Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms
- PMID: 40572502
- PMCID: PMC12195962
- DOI: 10.3390/molecules30122539
Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms
Abstract
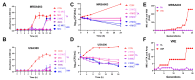
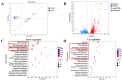
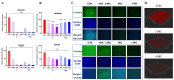
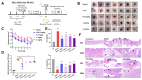
The emergence of methicillin-resistant Staphylococcus aureus (MRSA) and its biofilm-forming ability underscore the limitations of current antibiotics. In this study, a new compound named tripterhyponoid A was found to effectively combat MRSA, with an MIC of 2.0 μg/mL. It inhibited biofilm formation by downregulating genes related to the quorum sensing (QS) pathway (sarA, agrA, agrB, agrC, agrD, and hld) and eradicated mature biofilms. Furthermore, it induced DNA damage by binding to bacterial DNA, enhancing its efficiency against MRSA. Therefore, its anti-MRSA properties with multiple mechanisms of action make it less prone to developing resistance over 20 days. In addition, it reduced the bacterial load and regulated the levels of inflammatory cytokines IL-6 and IL-10 at the wound site in a mouse skin infection model. This paper provides the first in-depth investigation of the mechanisms of triterpenoids against MRSA by inhibiting the expression of QS system genes and binding to DNA.
Keywords: DNA binding; anti-MRSA; antibiofilm; quorum sensing; tripterhyponoid A.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

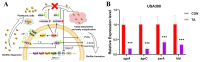


Similar articles
-
Deciphering the dynamics of methicillin-resistant Staphylococcus aureus biofilm formation: from molecular signaling to nanotherapeutic advances.Cell Commun Signal. 2024 Mar 22;22(1):188. doi: 10.1186/s12964-024-01511-2. Cell Commun Signal. 2024. PMID: 38519959 Free PMC article. Review.
-
Dextran guanidinylated carbon dots with antibacterial and immunomodulatory activities as eye drops for the topical treatment of MRSA-induced infectious keratitis.Acta Biomater. 2025 Jun 15;200:591-609. doi: 10.1016/j.actbio.2025.05.032. Epub 2025 May 13. Acta Biomater. 2025. PMID: 40374136
-
Antibiofilm efficacy of emodin alone or combined with ampicillin against methicillin-resistant Staphylococcus aureus.Sci Rep. 2025 Jul 1;15(1):21904. doi: 10.1038/s41598-025-06800-5. Sci Rep. 2025. PMID: 40596114 Free PMC article.
-
Oxidized chitosan: Combining an "Adhesion-and-Kill" antibacterial strategy with immunoregulation and angiogenesis to enhance methicillin-resistant Staphylococcus Aureus-infected wound healing.Int J Biol Macromol. 2025 Aug;319(Pt 4):145664. doi: 10.1016/j.ijbiomac.2025.145664. Epub 2025 Jun 30. Int J Biol Macromol. 2025. PMID: 40602574
-
Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis.Cochrane Database Syst Rev. 2018 Jul 21;7(7):CD009650. doi: 10.1002/14651858.CD009650.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Dec 13;12:CD009650. doi: 10.1002/14651858.CD009650.pub5. PMID: 30030966 Free PMC article. Updated.
References
-
- Naghavi M., Vollset S.E., Ikuta K.S., Swetschinski L.R., Gray A.P., Wool E.E., Aguilar G.R., Mestrovic T., Smith G., Han C., et al. Global burden of bacterial antimicrobial resistance 1990-2021: A systematic analysis with forecasts to 2050. Lancet. 2024;404:1199–1226. doi: 10.1016/S0140-6736(24)01867-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

