Nickel-Driven Electrochemical Upgrading of Kraft Lignin to Value-Added Aliphatic and Phenolic Products
- PMID: 40572509
- PMCID: PMC12195935
- DOI: 10.3390/molecules30122544
Nickel-Driven Electrochemical Upgrading of Kraft Lignin to Value-Added Aliphatic and Phenolic Products
Abstract
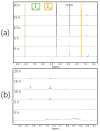
The depolymerization of lignin represents a promising strategy for its efficient utilization as a precursor for industrial raw materials. However, achieving both high efficiency and environmental sustainability remains a significant challenge. In this study, we present an aqueous electrochemical approach employing nickel as an electrocatalyst, enabling both depolymerization and partial de-aromatization of Kraft lignin under mild reaction conditions. Using an aqueous sodium carbonate medium, room temperature and ambient pressure, we achieved lignin depolymerization over reaction times ranging from 5 to 20 h. Characterization by nuclear magnetic resonance (NMR) spectroscopy confirmed the formation of aliphatic products such as acetate and formate, while high-resolution mass spectrometry (HRMS) confirmed the formation of a wide range of phenolic compounds. The conversion of lignin into valuable aromatic and aliphatic compounds offers a promising pathway for the synthesis of a wide range of organic chemicals and their subsequent industrial utilization, thereby supporting the development of a more sustainable economy.
Keywords: Green Chemistry; depolymerization; electrocatalysis; lignin; nickel.
Conflict of interest statement
Author Marcella Frauscher is employed by AC2T Research GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that the study received funding from the Austrian COMET program (Project InTribology2, No. 906860). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it.
Figures




References
-
- Crestini C., Lange H., Sette M., Argyropoulos D.S. On the Structure of Softwood Kraft Lignin. Green Chem. 2017;19:4104–4121. doi: 10.1039/C7GC01812F. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

