Photoexcited Palladium Complex-Catalyzed Isocyanide Insertion into Inactivated Alkyl Iodides
- PMID: 40572545
- PMCID: PMC12196398
- DOI: 10.3390/molecules30122584
Photoexcited Palladium Complex-Catalyzed Isocyanide Insertion into Inactivated Alkyl Iodides
Abstract
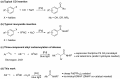
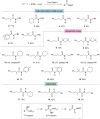
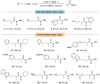
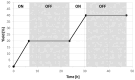
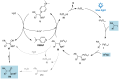
Isocyanides insertions represent an important transformation in the palladium-catalyzed reactions landscape. However, one of their most significant limitations is in the use of inactivated alkyl electrophiles. Palladium photocatalysis has been proven as a solid tool for the generation of alkyl radicals from alkyl halides, which may engage in subsequent transformations with a variety of reaction partners, closing the catalytic cycle. Herein, we report the mild three-component isocyanide insertions into inactivated alkyl iodides mediated by the catalytic activity of a photoexcited palladium complex. We investigated the scope of the reaction obtaining differently substituted secondary amides in good to high yields. We also investigated the mechanism, hypothesizing a key role of 4-(N,N-dimethylamino)pyridine in the outcome of the reaction.
Keywords: amide synthesis; isocyanide insertion; multicomponent reactions; palladium catalysis; photocatalysis; radical addition.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Barnard C.F.J. Palladium-Catalyzed Carbonylation—A Reaction Come of Age. Organometallics. 2008;27:5402–5422. doi: 10.1021/om800549q. - DOI
-
- Banfi L., Riva R., Basso A. Chiral Nonracemic Isocyanides. In: Nenajdenko V., editor. Isocyanide Chemistry: Applications in Synthesis and Material Science. Wiley-VCH; Weinheim, Germany: 2012. pp. 1–33.
LinkOut - more resources
Full Text Sources

