Multifunctional Evaluation of Graphene Oxide-Sulfonamide Nanoconjugates: Antimicrobial, Antibiofilm, Cytocompatibility and Xenobiotic Metabolism Gene Expression Insight
- PMID: 40572550
- PMCID: PMC12196119
- DOI: 10.3390/molecules30122585
Multifunctional Evaluation of Graphene Oxide-Sulfonamide Nanoconjugates: Antimicrobial, Antibiofilm, Cytocompatibility and Xenobiotic Metabolism Gene Expression Insight
Abstract
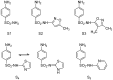
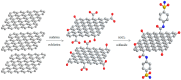
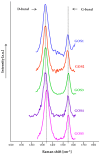
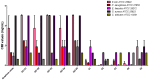
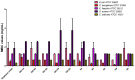
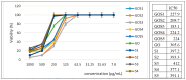
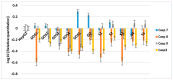
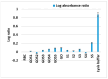
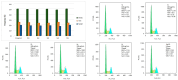
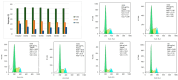
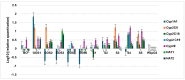
The clinical utility of sulfonamide antibiotics is increasingly challenged by antimicrobial resistance and pharmacokinetic limitations. In this study, we synthesized five graphene oxide-sulfonamide nanoconjugates (GO-S1 to GO-S5) via covalent functionalization, comprehensively characterized them by IR, Raman, SEM, EDS, etc., and evaluated their antimicrobial, antibiofilm, cytotoxic, apoptotic, hemolytic and gene expression-modulating effects. While the free sulfonamides (S1-S5) exhibited superior antimicrobial activity in planktonic cultures (MICs as low as 19 μg/mL), their GO-functionalized counterparts demonstrated enhanced antibiofilm efficacy, particularly against Pseudomonas aeruginosa (MBIC: 78-312 μg/mL). Cytotoxicity studies using CellTiter assays and Incucyte live-cell imaging revealed low toxicity for all compounds below 250 μg/mL. Morphological and gene expression analyses indicated mild pro-apoptotic effects, predominantly via caspase-9 and caspase-7 activation, with minimal caspase-3 involvement. Hemolysis assays confirmed the improved blood compatibility of GO-Sx conjugates compared to GO alone. Furthermore, qRT-PCR analysis showed that GO-Sx modulated the expression of key xenobiotic metabolism genes (CYPs and NATs), highlighting potential pharmacokinetic implications. Among all tested formulations, GOS3, GOS4 and GOS5 emerged as the most promising candidates, balancing low cytotoxicity, high hemocompatibility and strong antibiofilm activity. These findings support the use of graphene oxide nanocarriers to enhance the therapeutic potential of sulfonamides, particularly in the context of biofilm-associated infections.
Keywords: N-acetyltransferase expression; antimicrobial; apoptosis; cell cycle; cytochrome P450; graphene oxide; sulfonamide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Liposome-encapsulated antibiotics and biosurfactants: an effective strategy to boost biofilm eradication in cooling towers.Microb Cell Fact. 2025 Jun 18;24(1):135. doi: 10.1186/s12934-025-02746-5. Microb Cell Fact. 2025. PMID: 40533735 Free PMC article.
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2017 Oct 5;10(10):CD009528. doi: 10.1002/14651858.CD009528.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jun 10;6:CD009528. doi: 10.1002/14651858.CD009528.pub5. PMID: 28981972 Free PMC article. Updated.
-
Computational and experimental validation of 4-amino-3-hydroxynaphthalene-1-sulfonic acid as a novel antibiofilm agent.Eur J Pharmacol. 2025 Sep 15;1003:177892. doi: 10.1016/j.ejphar.2025.177892. Epub 2025 Jul 3. Eur J Pharmacol. 2025. PMID: 40617383
-
Targeting ESKAPE pathogens with ZnS and Au@ZnS Core-Shell nanoconjugates for improved biofilm control.Sci Rep. 2025 Jul 1;15(1):21407. doi: 10.1038/s41598-025-07583-5. Sci Rep. 2025. PMID: 40595152 Free PMC article.
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2012 Nov 14;11:CD009528. doi: 10.1002/14651858.CD009528.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Mar 05;(3):CD009528. doi: 10.1002/14651858.CD009528.pub3. PMID: 23152277 Updated.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

