Synthesis and Properties of 1 H-Pyrrolo[3',2':3,4]fluoreno[9,1- gh]quinolines and 7 H-Pyrrolo[2',3',4':4,10]anthra[1,9- fg]quinolines
- PMID: 40572578
- PMCID: PMC12195833
- DOI: 10.3390/molecules30122615
Synthesis and Properties of 1 H-Pyrrolo[3',2':3,4]fluoreno[9,1- gh]quinolines and 7 H-Pyrrolo[2',3',4':4,10]anthra[1,9- fg]quinolines
Abstract
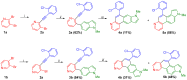
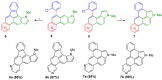
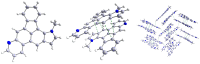
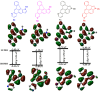
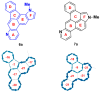
We report the synthesis of pyrrolo[3',2':3,4]fluoreno[9,1-gh]quinoline and pyrrolo[2',3',4':4,10]anthra[1,9-fg]quinoline derivatives. This novel class of N-doped polycyclic heteroaromatic compounds was synthesized by a site-selective cross-coupling reaction followed by acid-mediated cycloisomerization and Pd-catalyzed CH arylation as the final ring-closing reactions. Preliminary optical and aromatic properties were studied by means of steady-state absorption and fluorescence spectroscopy and DFT calculation. Special emphasis was placed on the impact of ring alternation and position of the N-doping within the scaffold.
Keywords: CH activation; PAHs; catalysis; cyclization; heterocycles; palladium; regioselectivity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
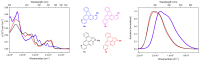
Similar articles
-
New Tools in Heavy Metal Detection: Synthesis, Spectroscopic, and Quantum Chemical Characterization of Selected Water-Soluble Styryl Derivatives of Quinoline and 1,10-Phenanthroline.Molecules. 2025 Jun 19;30(12):2659. doi: 10.3390/molecules30122659. Molecules. 2025. PMID: 40572623 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Ag(I) induced H-type and Pd(II) induced J-type phthalocyanines to enhance PDT applications: synthesis, optical behaviors, photochemical/photophysical properties, and DFT studies.Dalton Trans. 2025 Jun 24;54(25):10052-10070. doi: 10.1039/d5dt00590f. Dalton Trans. 2025. PMID: 40476833
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article.
References
-
- Abdel-Shafy H.I., Mansour M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016;25:107–123. doi: 10.1016/j.ejpe.2015.03.011. - DOI
-
- Li Q., Zhang Y., Xie Z., Zhen Y., Hu W., Dong H. Polycyclic aromatic hydrocarbon-based organic semiconductors: Ring-closing synthesis and optoelectronic properties. J. Mater. Chem. C. 2022;10:2411–2430. doi: 10.1039/D1TC04866J. - DOI
LinkOut - more resources
Full Text Sources

