Cobalt(II) Complexes of 4'-Nitro-Fenamic Acid: Characterization and Biological Evaluation
- PMID: 40572584
- PMCID: PMC12195967
- DOI: 10.3390/molecules30122621
Cobalt(II) Complexes of 4'-Nitro-Fenamic Acid: Characterization and Biological Evaluation
Abstract
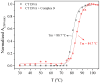
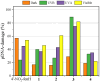
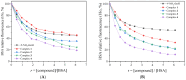
A nitro-derivative of fenamic acid (4'-nitro-fenamic acid) was synthesized and used as ligand for the synthesis of four Co(II) complexes in the absence or presence of the N,N'-donors 2,2'-bipyridylamine, 1,10-phenanthroline and 2,9-dimethyl-1,10-phenanthroline. The characterization of the resultant complexes was performed with diverse techniques (elemental analysis, molar conductivity measurements, IR and UV-vis spectroscopy, single-crystal X-ray crystallography). The biological evaluation of the compounds encompassed (i) antioxidant activity via hydrogen peroxide (H2O2) reduction and free radical scavenging; (ii) antimicrobial screening against two Gram-positive and two Gram-negative bacterial strains; (iii) interactions with calf-thymus (CT) DNA; (iv) cleavage of supercoiled pBR322 plasmid DNA (pDNA), in the dark or under UVA/UVB/visible light irradiation; and (v) binding affinity towards bovine and human serum albumins. The antioxidant activity of the compounds against 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radicals and H2O2 is significant, especially in the case of H2O2. The complexes exhibit adequate antimicrobial activity against the strains tested. The complexes interact with CT DNA through intercalation with binding constants reaching a magnitude of 106 M-1. The compounds have a significantly enhanced pDNA-cleavage ability under irradiation, showing promising potential as photodynamic therapeutic agents. All compounds can bind tightly and reversibly to both albumins tested.
Keywords: affinity for albumins; antibacterial activity; antioxidant activity; cobalt(II) complexes; fenamates; interaction with DNA; transition metal complexes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

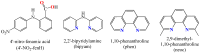




Similar articles
-
Structure and in vitro and in silico biological activity of zinc(II) complexes with 3,5-dichloro-salicylaldehyde.J Inorg Biochem. 2022 Apr;229:111727. doi: 10.1016/j.jinorgbio.2022.111727. Epub 2022 Jan 20. J Inorg Biochem. 2022. PMID: 35093777
-
Synthesis, Characterization and Biological Profile of Cationic Cobalt Complexes with First-Generation Quinolones.Molecules. 2025 Jun 19;30(12):2646. doi: 10.3390/molecules30122646. Molecules. 2025. PMID: 40572609 Free PMC article.
-
Synthesis, Characterization, Investigation of DNA Interactions and Biological Evaluation of Co(II), Ni(II), Cu(II) and Zn(II) Complexes with Newly Synthesized 2-methoxy 5-trifluoromethyl benzenamine Schiff Base.J Fluoresc. 2025 Jul;35(7):5301-5317. doi: 10.1007/s10895-024-03888-2. Epub 2024 Aug 31. J Fluoresc. 2025. PMID: 39215911
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
First trimester ultrasound tests alone or in combination with first trimester serum tests for Down's syndrome screening.Cochrane Database Syst Rev. 2017 Mar 15;3(3):CD012600. doi: 10.1002/14651858.CD012600. Cochrane Database Syst Rev. 2017. PMID: 28295158 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

