Phospholipase A2-A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom
- PMID: 40572586
- PMCID: PMC12196488
- DOI: 10.3390/molecules30122623
Phospholipase A2-A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom
Abstract
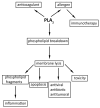
Phospholipase A2 (PLA2) is a prevalent molecule in the honeybee venom. Its importance is reflected by the number of scientists focused on studying it from various points of view. This review summarises a significant amount of data concerning this fascinating substance. Firstly, the origin and occurrence of PLA2, with similarities and differences among species or populations of bees are highlighted. Next, its synthesis, post-translational processing and structural features are described, followed by the PLA2 availability. In a larger section, the multiple effects of honeybee venom PLA2 are detailed, starting with the main ability as an enzyme to interact with biological membranes and to hydrolyse the sn-2 ester bond in 1,2-diacyl-sn-3-phosphoglycerides; the docking process, the substrate binding and the catalytic steps are analysed too. Then, the pro-/anti-inflammatory effect and allergenic property, the anticoagulant effect and the involvement of PLA2 in apoptosis are revised. Selected antiviral, antibiotic and antitumoral effects of PLA2, as well as its use in immunotherapy are mentioned as beneficial applications. Additionally, the mechanisms of toxicity of PLA2 are presented in detail. Finally, a number of anti-PLA2 compounds are enumerated. In each section, the features of the honeybee venom molecule are discussed in relation to PLA2s from other species.
Keywords: Apis mellifera; allergen; anti-PLA2 molecules; cell membrane; honeybee venom; phospholipase A2; phospholipids; toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

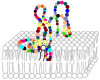
Similar articles
-
An Experimental Model of Acute Pulmonary Damage Induced by the Phospholipase A2-Rich Venom of the Snake Pseudechis papuanus.Toxins (Basel). 2025 Jun 12;17(6):302. doi: 10.3390/toxins17060302. Toxins (Basel). 2025. PMID: 40559880 Free PMC article.
-
A systematic review of the clinical effectiveness and cost-effectiveness of Pharmalgen® for the treatment of bee and wasp venom allergy.Health Technol Assess. 2012;16(12):III-IV, 1-110. doi: 10.3310/hta16120. Health Technol Assess. 2012. PMID: 22409877 Free PMC article.
-
The anticoagulant effect of Apis mellifera phospholipase A2 is inhibited by CORM-2 via a carbon monoxide-independent mechanism.J Thromb Thrombolysis. 2020 Jan;49(1):100-107. doi: 10.1007/s11239-019-01980-0. J Thromb Thrombolysis. 2020. PMID: 31679116
-
Combination of Rhamnetin and RXP03 Mitigates Venom-Induced Toxicity in Murine Models: Preclinical Insights into Dual-Target Antivenom Therapy.Toxins (Basel). 2025 Jun 4;17(6):280. doi: 10.3390/toxins17060280. Toxins (Basel). 2025. PMID: 40559858 Free PMC article.
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
References
-
- Bücherl W. Introduction. In: Bücherl W., Buckley E., editors. Venomous Animals and Their Venoms. Volume 1. Academic Press; New York, NY, USA: 1971. pp. xix–xxii. - DOI
-
- Maschwitz U.W.J., Kloft W. Morphology and function of the venom apparatus of insects—Bees, wasps, ants and caterpillars. In: Bücherl W., Buckley E., editors. Venomous Animals and Their Venoms. Volume 1. Academic Press; New York, NY, USA: 1971. pp. 1–60. - DOI
-
- Neumann W., Habermann E., Amend G. Zur Papierelektrophoresis den Fraktionierung tierischer Gifte. Naturwissenschaften. 1952;39:286–287. doi: 10.1007/BF00591257. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

