Optimization of Anthralin Microemulgel Targeted Delivery for Psoriasis and Acne
- PMID: 40572592
- PMCID: PMC12195956
- DOI: 10.3390/molecules30122629
Optimization of Anthralin Microemulgel Targeted Delivery for Psoriasis and Acne
Abstract
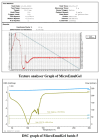
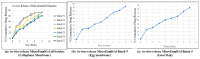
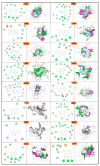
Background: Anthralin is known for its efficacy in treating psoriasis and acne, possessing poor solubility. Addressing these limitations, the present study endeavors to develop a microemulgel formulation of anthralin aimed at enhancing solubility. Method: The solubility study was performed in various solvents. An o/w (oil-in-water) emulsion was formed using the water titration method, which was optimized by statistical experimental design half-run CCD. The final optimized batch was evaluated for physicochemical and in vitro properties Result: The final optimized batch showed a particle size (PS) of 417 nm, -25.2 mV zeta potential (ZP) and pH 5.8, which remained stable upon centrifugation, heating-cooling and freeze-thawing cycle. Furthermore, microemulsion with Carbopol 943 5% w/v was selected as the gel base for the formation of microemulgel characterized by PS, ZP, pH, and viscosity of 230 nm, -50.6 mV, 6.9 and 14,200 cps, respectively, that ensured it a high enough stability. In silico molecular docking between ligand and protein provides the binding energies validating the interaction. Hence, the in silico study was performed for psoriasis and P. acne proteins. An in vitro antibacterial activity study on Propionibacterium revealed a significant efficiency of the formulation and MTT assay using L929 cell line in the presence of the drug-loaded microemulgel indicated an inhibition of growth proving that formulation has anti-psoriatic activity. Conclusions: Combination therapy with Clindamycin might improve efficacy while reducing antibiotic resistance risks.
Keywords: acne; anthralin; central-composite design; microemulgel; molecular docking; psoriasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
Polyherbal Antiacne Gel: In Vitro Antibacterial Activity and Efficacy Evaluation Against Cutibacterium acnes.Assay Drug Dev Technol. 2024 Oct;22(7):373-386. doi: 10.1089/adt.2024.031. Epub 2024 Sep 10. Assay Drug Dev Technol. 2024. PMID: 39253845
-
Minocycline for acne vulgaris: efficacy and safety.Cochrane Database Syst Rev. 2003;(1):CD002086. doi: 10.1002/14651858.CD002086. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD002086. doi: 10.1002/14651858.CD002086.pub2. PMID: 12535427 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Betulin-NLC-hydrogel for the Treatment of Psoriasis-like Skin Inflammation: Optimization, Characterisation, and In vitro and In vivo Evaluation.Curr Drug Deliv. 2025;22(5):627-647. doi: 10.2174/0115672018329544240922151617. Curr Drug Deliv. 2025. PMID: 39360545
References
-
- Laochunsuwan A., Taweechotipatr M., Udompataikul M. In Vitro Study of Antibiotic Susceptibility of Propionibacterium Acnes Strains Isolated from Acne Vulgaris Patients. J. Med. Assoc. Thai. 2017;100:S24–S31.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

