Synthesis of 1-(2-Hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as a Promising Scaffold Against Disease-Causing Bacteria Relevant to Public Health
- PMID: 40572602
- PMCID: PMC12196313
- DOI: 10.3390/molecules30122639
Synthesis of 1-(2-Hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as a Promising Scaffold Against Disease-Causing Bacteria Relevant to Public Health
Abstract
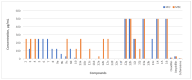
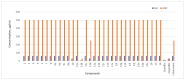
The increasing number of antibiotic-resistant pathogens forces us to accelerate the search for new antimicrobial agents. Based on this, we chose to synthesize a library of 1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic acid derivatives and evaluate their antibacterial activity against various pathogens. A series of (2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic acid and its hydrazide derivatives were prepared and identified by the methods of IR, 1H, and 13C NMR spectroscopy and a microanalysis technique. The resulting compounds were evaluated in vitro for their efficacy against the Gram-positive Staphylococcus aureus (ATCC 9144), Listeria monocytogenes (ATCC 7644), and Bacillus cereus (ATCC 11778) bacterial strains as well as the Gram-negative Escherichia coli (ATCC 8739) bacteria. Oxacillin, ampicillin, and cefuroxime were used as control antibiotics. Among the obtained compounds, hydrazone with a 5-nitrothien-2-yl fragment surpassed the control cefuroxime (7.8 μg/mL) against almost all strains tested. Hydrazone with a 5-nitrofuran-2-yl moiety showed a slightly lower but also potent effect on all bacterial strains. Moreover, hydrazone with a benzylidene moiety demonstrated very strong inhibition of S. aureus (3.9 μg/mL) in comparison with the antibacterial drug cefuroxime (7.8 μg/mL). In addition, some of these compounds exhibited remarkable bactericidal properties. In a complete biofilm disruption study, 5-nitrothienylhydrazone showed excellent results in disrupting S. aureus and E. coli biofilms. The test results show the potential of the newly obtained derivatives as a source of antibacterial agents. Therefore, further studies on the molecular optimization of these compounds are necessary for the development of new antibacterial drugs.
Keywords: 5-oxopyrrolidine; antibacterial activity; biofilm formation; heterocycles; hydrazone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Borah P., Hazarika S., Chettri A., Sharma D., Deka S., Venugopala K.N., Shinu P., Al-Shar’i N.A., Bardaweel S.K., Deb P.K. Viral, Parasitic, Bacterial, and Fungal Infections. Academic Press; Cambridge, MA, USA: 2023. Heterocyclic compounds as antimicrobial agents; pp. 781–804. - DOI
-
- Qadir T., Amin A., Sharma P.K., Jeelani I., Abe H. A Review on Medicinally Important Heterocyclic Compounds. Open J. Med. Chem. 2022;16:e187410452202280. doi: 10.2174/18741045-v16-e2202280. - DOI
-
- Anwer K.E., El-Hddad S.S.A., Abd El-Sattar N.E.A., El-Morsy A., Khedr F., Mohamady S., Keshek D.E., Salama S.A., El-Adl K., Hanafy N.S. Five and Six Membered Heterocyclic Rings Endowed with Azobenzene as Dual EGFRT790M and VEGFR-2 Inhibitors: Design, Synthesis, in Silico ADMET Profile, Molecular Docking, Dynamic Simulation and Anticancer Evaluations. RSC Adv. 2023;13:35321–35338. doi: 10.1039/D3RA06614B. - DOI - PMC - PubMed
-
- Kabir E., Monir Uzzaman M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022;4:100606. doi: 10.1016/j.rechem.2022.100606. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

