HER2 in Non-Small Cell Lung Cancer (NSCLC): Evolution of the Therapeutic Landscape and Emerging Drugs-A Long Way to the Top
- PMID: 40572608
- PMCID: PMC12195848
- DOI: 10.3390/molecules30122645
HER2 in Non-Small Cell Lung Cancer (NSCLC): Evolution of the Therapeutic Landscape and Emerging Drugs-A Long Way to the Top
Abstract
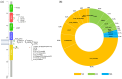
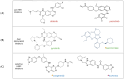
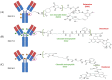
Non-small-cell lung cancer (NSCLC) can harbour different HER2 alterations: HER2 protein overexpression (2-35%), HER2 gene amplification (2-20%), and gene mutations (1-4%). The discovery of the HER2 gene in the 1980s raised great expectations for the treatment of several tumours. However, it was only in 2004 that HER2 mutations were identified, and they currently represent a key druggable target in NSCLC. Despite numerous strengths, there is only one FDA/EMA-approved targeted therapy, an antibody-drug conjugate (ADC) called trastuzumab deruxtecan for pretreated patients with HER2 mutant NSCLC. In the first-line treatment, the standard of care (SoC) remains chemotherapy with or without immunotherapy. In the past, pan-HER tyrosine kinase inhibitors (TKIs) were extensively studied with poor results. But, two newly developed HER2-specific TKIs with low EGFR WT inhibition (BAY2927088 and zongertinib) reported encouraging results and received the breakthrough therapy designation from the FDA. Ongoing clinical trials are investigating new agents. This review focuses on HER2 alterations. Additionally, the anti-HER2 therapies explored so far will be discussed in detail, including the following: HER2 inhibitors (pan-inhibitors and selective inhibitors), monoclonal antibodies (mAbs), and ADCs. A section of this paper is dedicated to the role of immunotherapy in HER2-altered NSCLC. The last section of this paper focuses on the drugs under development and their challenges.
Keywords: ADC; ERBB2; HER2; NSCLC; adenocarcinoma; antibody–drug conjugate; immunotherapy; targeted therapy; tyrosine kinase inhibitor.
Conflict of interest statement
G.S. served as an advisor for Takeda outside of the submitted work. I.A. received consulting fees (advisory board) from Bristol-Myers Squibb, Johnson&Johnson. A.P. served as a consultant or advisor for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche/Genentech and received speaker fees from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Jansen, Eli Lilly, Merck Sharp & Dohme, e Cancer, and Medscape—all outside of the submitted work. F.d.M. has received advisory fees from Roche, Bristol-Myers Squibb, and AstraZeneca and consulting fees from Merck Sharp & Dohme—all outside of the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Erlotinib or gefitinib for the treatment of relapsed platinum pretreated non-small cell lung cancer and ovarian cancer: a systematic review.Drug Resist Updat. 2011 Jun;14(3):177-90. doi: 10.1016/j.drup.2011.02.004. Epub 2011 Mar 24. Drug Resist Updat. 2011. PMID: 21435938
-
Zongertinib in Previously Treated HER2-Mutant Non-Small-Cell Lung Cancer.N Engl J Med. 2025 Jun 19;392(23):2321-2333. doi: 10.1056/NEJMoa2503704. Epub 2025 Apr 28. N Engl J Med. 2025. PMID: 40293180 Clinical Trial.
-
Prevalence and Therapeutic Targeting of High-Level ERBB2 Amplification in NSCLC.J Thorac Oncol. 2024 May;19(5):732-748. doi: 10.1016/j.jtho.2023.12.019. Epub 2023 Dec 26. J Thorac Oncol. 2024. PMID: 38154514 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.Cochrane Database Syst Rev. 2016 May 25;(5):CD010383. doi: 10.1002/14651858.CD010383.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD010383. doi: 10.1002/14651858.CD010383.pub3. PMID: 27223332 Updated.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

