Long COVID and Biomarker Dysregulation-A Shift Toward Immune Exhaustion?
- PMID: 40572685
- PMCID: PMC12195428
- DOI: 10.3390/medicina61060996
Long COVID and Biomarker Dysregulation-A Shift Toward Immune Exhaustion?
Abstract
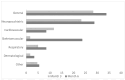
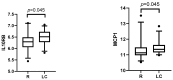
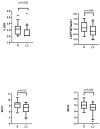
Background: SARS-CoV-2 infection can lead to persistent or newly emerging symptoms lasting for months, a condition known as long COVID (LC). The pathophysiology of LC remains poorly understood, with cytokine dysregulation proposed as a key mechanism, although findings across the studies have been inconsistent. Patients and methods: We conducted a longitudinal study using the Olink® Target 96 Inflammation Panel to assess cytokines in COVID-19 (COV) patients at three months and six months post-infection. These profiles were compared with those of individuals recovering from other upper respiratory tract infections (non-COV). Additionally, we analyzed differences between individuals with LC and those who recovered from COVID-19. Predictive models for LC at three months and sixth months post-infection were developed using inflammatory markers and relevant clinical cofactors, including gender, age, BMI, hemogram, Β2-microglobulin, D-dimers, LDH, AST, ALT, Ferritin, vitamin D, CRP, and the severity of acute COVID-19 infection as classified by WHO criteria. Results: We observed a general decline in inflammatory biomarkers in post-COVID-19 patients over time, with only a few cytokines elevated (CCL4 at month 3 and CST5 at month 6) compared to non-COV controls. In LC patients, an early phase of low-grade inflammation transitioned into significant reduction in proinflammatory biomarkers compared to recovered individuals. Rather than indicating immune normalization, this pattern suggests a possible suppression or exhaustion of the immune response in the months following acute infection. Importantly, our predictive modeling demonstrated that this specific cytokine signature, in combination with acute disease severity and clinical cofactors, described well the presence of LC. Conclusions: Our findings suggest that inflammation-related biomarker dysregulation following acute SARS-CoV-2 infection evolves dynamically over a six-month period. By the sixth month, compared to the third month, the presence of LC is more accurately predicted by a combination of persistent biomarker alteration and the severity of the initial infection, as defined by WHO criteria. This represents a novel insight, as previous studies have primarily associated LC with elevated proinflammatory markers, whereas our results suggest that immune suppression or exhaustion may play a more prominent role in the later stages.
Keywords: Olink proteomics; SARS-CoV-2; inflammation; long COVID; post-COVID-19.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- World Health Organization . A Clinical Casedefinition of Post COVID-19 Condition by a Delphiconsensus. WHO; Geneva, Switzerland: 2021. [(accessed on 25 April 2025)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_cond....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

