Overview on the Thermally Activated Delayed Fluorescence and Mechanochromic Materials: Bridging Efficiency and Versatility in LECs and OLEDs
- PMID: 40572847
- PMCID: PMC12194274
- DOI: 10.3390/ma18122714
Overview on the Thermally Activated Delayed Fluorescence and Mechanochromic Materials: Bridging Efficiency and Versatility in LECs and OLEDs
Abstract
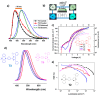
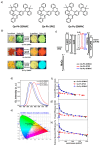
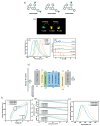
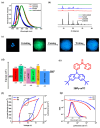
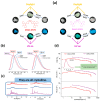
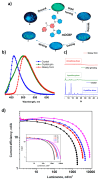
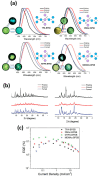
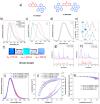
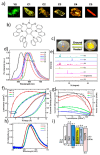
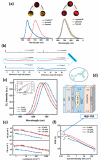
Recent advancements in thermally activated delayed fluorescence (TADF) materials and mechanochromic materials have significantly enhanced the efficiency and versatility of light-emitting electrochemical cells (LECs) and organic light-emitting diodes (OLEDs). TADF materials have enabled efficiency improvements, achieving an internal quantum efficiency (IQE) of nearly 100% by utilizing both singlet and triplet excitons. Meanwhile, mechanochromic materials exhibit reversible optical changes upon mechanical stimuli, making them promising for stress sensing, encryption, and flexible electronics. The synergistic integration of TADF and mechanochromic materials in OLEDs and LECs has led to enhanced efficiency, stability, and multifunctionality in next-generation lighting and display technologies. This narrative review explores recent breakthroughs in devices that incorporate both TADF and mechanochromic materials as emitters. Particular attention is given to the molecular design that enable both TADF and mechanochromic properties, as well as optimal device structures and performance parameters. Moreover, this review discusses the only LEC fabricated so far using a TADF-mechanochromic emitter, highlighting its performance and potential. Finally, the report concludes with an outlook on the future commercial applications of these materials, particularly in wearable electronics and smart display technologies.
Keywords: light-emitting electrochemical cells (LECs); mechanochromic; organic light-emitting diodes (OLEDs); reverse intersystem crossing (RISC); thermally activated delayed fluorescence (TADF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Modulating circularly polarized luminescent and thermally activated delayed fluorescence properties by introducing chiral unit and extending acceptor unit strategies.Spectrochim Acta A Mol Biomol Spectrosc. 2026 Jan 5;344(Pt 1):126710. doi: 10.1016/j.saa.2025.126710. Epub 2025 Jul 18. Spectrochim Acta A Mol Biomol Spectrosc. 2026. PMID: 40700904
-
Narrow-Band Dibenzoselenophene-Based Emitter with Rapid Triplet Conversion for Versatile OLED Applications with Superior Roll-Off Suppression.Angew Chem Int Ed Engl. 2025 Jul;64(29):e202507626. doi: 10.1002/anie.202507626. Epub 2025 May 16. Angew Chem Int Ed Engl. 2025. PMID: 40345985
-
Stable Pincer Gold(III)-TADF Emitters with Extended Donor-Acceptor Separation for Efficient Vacuum-Deposited OLEDs with Operational Lifetime (LT95) up to 3831 h at 1000 cd m-2.Adv Sci (Weinh). 2025 Jul;12(27):e2502529. doi: 10.1002/advs.202502529. Epub 2025 Apr 26. Adv Sci (Weinh). 2025. PMID: 40285612 Free PMC article.
-
Comprehensive Review on the Structural Diversity and Versatility of Multi-Resonance Fluorescence Emitters: Advance, Challenges, and Prospects toward OLEDs.Chem Rev. 2025 Jul 23;125(14):6685-6752. doi: 10.1021/acs.chemrev.5c00021. Epub 2025 May 9. Chem Rev. 2025. PMID: 40344420 Free PMC article. Review.
-
Phosphor-Assisted TADF-Sensitized Fluorescence (pTSF) OLEDs: Faster Excitons, Brighter Futures.Chemistry. 2025 Jul 2;31(37):e202501500. doi: 10.1002/chem.202501500. Epub 2025 May 28. Chemistry. 2025. PMID: 40393923 Review.
References
-
- Nakanotani H., Tsuchiya Y., Adachi C. Thermally-activated Delayed Fluorescence for Light-emitting Devices. Chem. Lett. 2021;50:938–948. doi: 10.1246/cl.200915. - DOI
-
- Dias F.B., Bourdakos K.N., Jankus V., Moss K.C., Kamtekar K.T., Bhalla V., Santos J., Bryce M.R., Monkman A.P. Triplet Harvesting with 100% Efficiency by Way of Thermally Activated Delayed Fluorescence in Charge Transfer OLED Emitters. Adv. Mater. 2013;25:3707–3714. doi: 10.1002/adma.201300753. - DOI - PubMed
-
- Zhao K., Zhao Y., Qian R., Ye C., Song Y. Recent Advances in Interactive Mechanosensory Electronics with Luminescence/Coloration Outputs for Wearable Applications. ACS Mater. Lett. 2023;5:3093–3116. doi: 10.1021/acsmaterialslett.3c00800. - DOI
-
- Weder C. Encyclopedia of Polymeric Nanomaterials. Springer; Berlin/Heidelberg, Germany: 2013. Mechanochromic Polymers; pp. 1–11. - DOI
-
- Dos Santos J.M., Hall D., Basumatary B., Bryden M., Chen D., Choudhary P., Comerford T., Crovini E., Danos A., De J., et al. The Golden Age of Thermally Activated Delayed Fluorescence Materials: Design and Exploitation. Chem. Rev. 2024;124:13736–14110. doi: 10.1021/acs.chemrev.3c00755. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

