NIR-Responsive Microbubble Delivery Platforms for Controlled Drug Release in Cancer Therapy
- PMID: 40572858
- PMCID: PMC12194853
- DOI: 10.3390/ma18122725
NIR-Responsive Microbubble Delivery Platforms for Controlled Drug Release in Cancer Therapy
Abstract
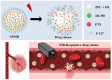
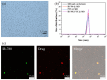
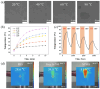
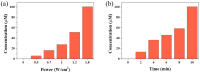
Cancer remains one of the leading causes of death worldwide. Therefore, the continuous development of effective therapeutic strategies is necessary. Conventional anticancer chemotherapy has low bioavailability and poor systemic distribution, resulting in serious side effects and limited therapeutic efficacy. To address these limitations, drug delivery systems that respond to external stimuli have been developed to release drugs at specific sites. In this study, a phase transition-based bubble-mediated emulsion system was developed to enable near-infrared (NIR)-induced drug release. This system consists of an oil phase, 2H,3H-perfluoropentane (PFC), a fluorinated liquid gas that evaporates at a certain temperature, and encapsulated IR-780 and paclitaxel to maintain stable microbubbles. Under NIR irradiation, IR-780 exhibits a photothermal conversion effect, which increases the temperature. Above the critical temperature, PFC undergoes a phase transition into gas, forming gas bubbles. This phase transition leads to a rapid volume expansion, destroys the microbubble structure, and triggers drug release. The NIR-responsive microbubble system developed in this study facilitated targeted and selective drug release through precise temperature control using the photothermal effects and phase transition. This system provides a novel platform to improve the efficacy of cancer therapies.
Keywords: cancer therapy; controlled release; drug delivery system; microbubble.
Conflict of interest statement
Myoung-Hwan Park is the CEO of N to B Co., Ltd., and Jungmin Lee and Bin Yoon are employees of N to B Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Cariolou M., Abar L., Aune D., Balducci K., Becerra-Tomas N., Greenwood D.C., Markozannes G., Nanu N., Vieira R., Giovannucci E.L., et al. Postdiagnosis recreational physical activity and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. Int. J. Cancer. 2023;152:600–615. doi: 10.1002/ijc.34324. - DOI - PMC - PubMed
-
- Nikolaos T. Obesity and Lung Cancer (Investigating the Relationship) EPRA Int. J. Multidiscip. Res. 2023;9:175–177. doi: 10.36713/epra12422. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

