The Potential of MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) as Adsorbents for the Efficient Separation of CH4 from CO2 and H2S
- PMID: 40573039
- PMCID: PMC12195146
- DOI: 10.3390/ma18122907
The Potential of MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) as Adsorbents for the Efficient Separation of CH4 from CO2 and H2S
Abstract
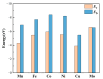
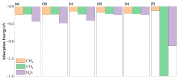
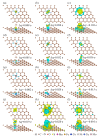
Carbon dioxide (CO2) and hydrogen sulfide (H2S) as harmful gases are always associated with methane (CH4) in natural gas, biogas, and landfill gas. Given that chemisorption and physisorption are the key gas separation technologies in industry, selecting appropriate adsorbents is crucial to eliminate these harmful gases. The adsorption of CH4, CO2, and H2S has been studied based on the density functional theory (DFT) in this work to evaluate the feasibility of transition metal (M = Mn, Fe, Co, Ni, Cu, Mo) porphyrin-like moieties embedded in graphene sheets (MN4-GPs) as adsorbents. It was found that the interactions between gas molecules and MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) are different. The weaker interactions between CH4 and MN4-GPs (M = Co, Ni, Cu, Mo) than those between CO2 and MN4-GPs or between H2S and MN4-GPs are beneficial to the separation of CH4 from CO2 and H2S. The maximum difference in the interactions between gas molecules and MoN4-GPs means that MoN4-GPs have the greatest potential to become adsorbents. The different interfacial interactions are related to the amount of charge transfer, which could promote the formation of bonds between gas molecules and MN4-GPs to effectively enhance the interfacial interactions.
Keywords: carbon dioxide; density functional theory; hydrogen sulfide; methane; transition metal porphyrin-like moieties embedded in graphene sheets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Prophylactic non-steroidal anti-inflammatory drugs for the prevention of macular oedema after cataract surgery.Cochrane Database Syst Rev. 2016 Nov 1;11(11):CD006683. doi: 10.1002/14651858.CD006683.pub3. Cochrane Database Syst Rev. 2016. PMID: 27801522 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
-
Effect of Ni and Fe Doping Levels on the Nano Core-Shell Structure La2Ni2-xFexO6@CeO2 Double Perovskite Type Composite Catalysts for Dry Reforming of Methane Performance.ACS Appl Mater Interfaces. 2025 Jul 2;17(26):37987-38003. doi: 10.1021/acsami.5c06220. Epub 2025 Jun 21. ACS Appl Mater Interfaces. 2025. PMID: 40542718
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
References
-
- Aghel B., Behaein S., Wongwises S., Shadloo M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy. 2022;160:106422. doi: 10.1016/j.biombioe.2022.106422. - DOI
-
- Wen X., Bai P., Luo B., Zheng S., Chen C. Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros. Sci. 2018;139:124–140. doi: 10.1016/j.corsci.2018.05.002. - DOI
Grants and funding
- RC202007/Leshan Normal University Doctoral Talent Launch Project
- 2022CHXK005/Open Project of Crystal Silicon Photovoltaic New Energy Research Institute
- KYPY2024-0001/Leshan Normal University Scientific Research Cultivation Project
- 2023CL10/Opening Project of Material Corrosion and Protec-tion Key Laboratory of Sichuan province
- RC2024029/Scientific Research Initiation Project for the Introduction of High-level Talents of Leshan Normal University
LinkOut - more resources
Full Text Sources

