Alginate Oligosaccharide and Gut Microbiota: Exploring the Key to Health
- PMID: 40573088
- PMCID: PMC12196054
- DOI: 10.3390/nu17121977
Alginate Oligosaccharide and Gut Microbiota: Exploring the Key to Health
Abstract
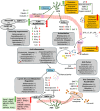
Alginate oligosaccharide (AOS), a degradation product of alginate derived from marine brown algae, has attracted significant attention due to its potent ability to modulate gut microbiota and enhance human health. This review aims to systematically introduce current evidence on the interactions between AOS and gut microbial communities, focusing on how AOS improves health through regulating gut microbiota. Initially, the structural factors of AOS that influence their functions are highlighted, including molecular weight, monomer composition, terminal structure, and chemical modifications. Importantly, AOS primarily exerts beneficial effects by adjusting gut microbiota community and outputs, which include the promotion of probiotics, the inhibition of pathogens, the balance of microbiota composition, and the increase of short-chain fatty acid production. Moreover, the discovered mechanisms underlying AOS-mediated health promotion via microbiota modulation are detailed comprehensively, specifically emphasizing intestinal barrier maintenance, antioxidation, dual-regulation of immune and inflammatory responses, pathogenic infection inhibition, metabolic improvement, uric acid excretion promotion, anti-tumor effects, and anti-skin aging. Such beneficial effects make AOS valuable in keeping healthy, preventing disorders, and intervening in diseases. Despite these findings and research progress, there are yet limitations in studying AOS-gut microbiota interactions, such as precise microbiota-targeted structural optimization, personalized nutritional interventions based on microbial characteristics, and broadening the horizon of microbiota-derived metabolic metabolomic profiles. In conclusion, advancing our understanding of the gut microbiota-centered mechanisms of AOS would probably facilitate novel nutritional strategy development for health promotion.
Keywords: alginate; gut microbiota; intestinal health; oligosaccharide; prebiotics; short-chain fatty acids; α-L-guluronic acid; β-D-mannuronic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The interplay of gut microbiota and intestinal motility in gastrointestinal function.J Smooth Muscle Res. 2025;61:51-58. doi: 10.1540/jsmr.61.51. J Smooth Muscle Res. 2025. PMID: 40619214 Free PMC article. Review.
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
-
Gut Microbiota-Targeted Therapeutics for Metabolic Disorders: Mechanistic Insights into the Synergy of Probiotic-Fermented Herbal Bioactives.Int J Mol Sci. 2025 Jun 7;26(12):5486. doi: 10.3390/ijms26125486. Int J Mol Sci. 2025. PMID: 40564947 Free PMC article. Review.
-
Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review.Nutrients. 2022 Jun 21;14(13):2559. doi: 10.3390/nu14132559. Nutrients. 2022. PMID: 35807739 Free PMC article.
-
Iota-carrageenan oligosaccharide ameliorates DSS-induced colitis in mice by mediating gut microbiota dysbiosis and modulating SCFAs-PI3K-AKT pathway.Inflammopharmacology. 2025 Jun;33(6):3443-3460. doi: 10.1007/s10787-025-01718-w. Epub 2025 Apr 1. Inflammopharmacology. 2025. PMID: 40167852
References
-
- Larsson E., Tremaroli V., Lee Y.S., Koren O., Nookaew I., Fricker A., Nielsen J., Ley R.E., Bäckhed F. Analysis of Gut Microbial Regulation of Host Gene Expression along the Length of the Gut and Regulation of Gut Microbial Ecology through MyD88. Gut. 2012;61:1124–1131. doi: 10.1136/gutjnl-2011-301104. - DOI - PMC - PubMed
-
- Shulzhenko N., Morgun A., Hsiao W., Battle M., Yao M., Gavrilova O., Orandle M., Mayer L., Macpherson A.J., McCoy K.D., et al. Crosstalk between B Lymphocytes, Microbiota and the Intestinal Epithelium Governs Immunity versus Metabolism in the Gut. Nat. Med. 2011;17:1585–1593. doi: 10.1038/nm.2505. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

