An Adapted Cardioprotective Diet with or Without Phytosterol and/or Krill Oil Supplementation in Familial Hypercholesterolemia: Results of a Pilot Randomized Clinical Trial
- PMID: 40573119
- PMCID: PMC12196534
- DOI: 10.3390/nu17122008
An Adapted Cardioprotective Diet with or Without Phytosterol and/or Krill Oil Supplementation in Familial Hypercholesterolemia: Results of a Pilot Randomized Clinical Trial
Abstract
Background/Objectives: Familial hypercholesterolemia (FH) is an increasingly common inherited disorder that increases cardiovascular risk. Despite the importance of lifestyle interventions, adherence to a healthy diet among individuals with FH remains suboptimal. This pilot, multicenter, double-blind, placebo-controlled randomized trial aimed to evaluate the feasibility and preliminary effects of a culturally adapted cardioprotective diet (DICA-FH), alone or in combination with phytosterol and/or krill oil supplementation, on lipid parameters in Brazilian adults with probable or definitive FH. Methods: Between May and August 2023, 58 participants were enrolled across nine Brazilian centers and randomized (1:1:1:1) into four groups: DICA-FH + phytosterol placebo + krill oil placebo; DICA-FH + phytosterol 2 g/day + krill oil placebo; DICA-FH + phytosterol placebo + krill oil 2 g/day; and DICA-FH + phytosterol 2 g/day + krill oil 2 g/day. Interventions lasted 120 days. The primary outcomes were mean low-density lipoprotein cholesterol (LDL-c) and lipoprotein(a) (Lp[a]) levels, as well as adherence to treatment at follow-up. Secondary outcomes included mean levels of other lipids, frequency of adverse events, and assessment of protocol implementation components. All data were presented separately for the allocation groups: phytosterol vs. placebo and krill oil vs. placebo. Results: Mean age was 54.5 ± 13.7 years, and 58.6% were women. Both adherence to protocol (91.8% attendance; 79.1% investigational product intake) and retention (86.2%) were high. No significant differences between groups were found for LDL-c or Lp(a). However, regardless of allocation to active supplementation or placebo, a significant reduction in Lp(a) concentrations was observed following the DICA-FH intervention (median difference: -3.8 mg/dL [interquartile range: -7.5 to -1.2]; p < 0.01). Significant reductions in oxidized LDL (LDL-ox) and LDL-ox/LDL-c ratio were also observed in the overall sample (p < 0.01). Although not statistically significant, all groups showed improvements in diet quality after 120 days. No serious adverse events related to the interventions were reported. Additionally, most protocol implementation components were successfully achieved. Conclusions: The DICA-FH strategy, with or without supplementation, was safe and well-tolerated. Although not powered to detect clinical efficacy (which is acceptable in exploratory pilot trials), the study supports the feasibility of a larger trial and highlights the potential of dietary interventions in the management of HF.
Keywords: diet; fatty acids; healthy; hypercholesterolemia; omega-3; phytosterols; pilot projects.
Conflict of interest statement
Author Julia Pinheiro Krey was employed by Hcor Nutrition Service. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
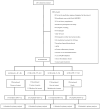
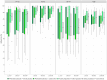
References
-
- Ferrières J., Banks V., Pillas D., Giorgianni F., Gantzer L., Lekens B., Désaméricq G. Screening and treatment of familial hypercholesterolemia in a French sample of ambulatory care patients: A retrospective longitudinal cohort study. PLoS ONE. 2021;16:e0255345. doi: 10.1371/journal.pone.0255345. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

