Alistipes putredinis Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease in Rats via Gut Microbiota Remodeling and Inflammatory Suppression
- PMID: 40573124
- PMCID: PMC12196099
- DOI: 10.3390/nu17122013
Alistipes putredinis Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease in Rats via Gut Microbiota Remodeling and Inflammatory Suppression
Abstract
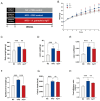
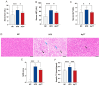
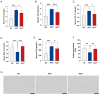
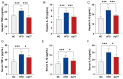
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a highly prevalent chronic liver condition linked to obesity and metabolic imbalance. Alterations in the gut microbiota are increasingly recognized as contributors to its progression. Alistipes putredinis, a core member of the human gut microbiota, has been linked with metabolic health, but its functional role in MASLD remains unclear. Methods: This study evaluated the potential of A. putredinis strain Ap77, isolated from the stool of a healthy adult, to mitigate MASLD-related alterations in a high-fat diet (HFD)-induced rat model. Animals were divided into normal chow (NC), HFD, and HFD plus Ap77 groups and received daily oral gavage of Ap77 or PBS for 8 weeks. Results: Ap77 supplementation attenuated the body weight increase associated with high-fat diet consumption. It also reduced hepatic triglyceride levels and fat mass and improved liver histology. Transcriptomic analysis revealed suppression of inflammation-associated pathways. Correspondingly, the concentrations of IL-1β, IL-6, and TNF-α in both the liver and serum were reduced. Ap77 supplementation was associated with an increased abundance of health-associated bacterial genera, such as Lachnospiraceae UCG_010, Akkermansia, and Flavonifractor, as well as elevated serum levels of butyrate, indole-3-propionic acid, and indoleacrylic acid. Notably, correlation analysis revealed that Lachnospiraceae UCG_010 was positively associated with these metabolites. Conclusions:A. putredinis Ap77 alleviates hepatic steatosis and inflammation in MASLD, potentially by reshaping gut microbiota and suppressing inflammation-related signaling pathways.
Keywords: Alistipes putredinis; MASLD; butyrate; gut microbiota; indole derivatives; inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Alisol B ameliorated metabolic dysfunction-associated steatotic liver disease via regulating purine metabolism and restoring the gut microbiota disorders.Phytomedicine. 2025 Sep;145:156992. doi: 10.1016/j.phymed.2025.156992. Epub 2025 Jun 22. Phytomedicine. 2025. PMID: 40582207
-
Phocaeicola dorei ameliorates progression of steatotic liver disease by regulating bile acid, lipid, inflammation and proliferation.Gut Microbes. 2025 Dec;17(1):2539448. doi: 10.1080/19490976.2025.2539448. Epub 2025 Aug 3. Gut Microbes. 2025. PMID: 40754863 Free PMC article.
-
Sodium butyrate ameliorates liver fibrosis in metabolic dysfunction-associated steatohepatitis rats via miR-155-5p/SOCS1/PDGF signaling pathway.Hepatobiliary Pancreat Dis Int. 2025 Aug;24(4):423-432. doi: 10.1016/j.hbpd.2025.04.006. Epub 2025 Apr 30. Hepatobiliary Pancreat Dis Int. 2025. PMID: 40360368
-
Gut-Liver Axis: The Role of Intestinal Microbiota and Their Metabolites in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease.Gut Liver. 2025 Jul 15;19(4):479-507. doi: 10.5009/gnl240539. Epub 2025 May 8. Gut Liver. 2025. PMID: 40336226 Free PMC article. Review.
-
Inappropriate Diet Exacerbates Metabolic Dysfunction-Associated Steatotic Liver Disease via Abdominal Obesity.Nutrients. 2024 Dec 5;16(23):4208. doi: 10.3390/nu16234208. Nutrients. 2024. PMID: 39683601 Free PMC article.
References
-
- Kanwal F., Neuschwander-Tetri B.A., Loomba R., Rinella M.E. Metabolic Dysfunction–Associated Steatotic Liver Disease: Update and Impact of New Nomenclature on the American Association for the Study of Liver Diseases Practice Guidance on Nonalcoholic Fatty Liver Disease. Hepatology. 2024;79:1212. doi: 10.1097/HEP.0000000000000670. - DOI - PubMed
-
- Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.-A. The Prevalence and Incidence of NAFLD Worldwide: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

