Huanglian Jiedu Decoction Treats Ischemic Stroke by Regulating Pyroptosis: Insights from Multi-Omics and Drug-Target Relationship Analysis
- PMID: 40573172
- PMCID: PMC12195757
- DOI: 10.3390/ph18060775
Huanglian Jiedu Decoction Treats Ischemic Stroke by Regulating Pyroptosis: Insights from Multi-Omics and Drug-Target Relationship Analysis
Abstract
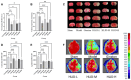
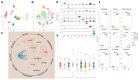
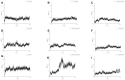
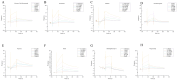
Background: Ischemic stroke (IS) is a severe condition with limited therapeutic options. Pyroptosis, a type of programmed cell death linked to inflammation, is closely associated with IS-related damage. Studies suggest inflammation aligns with the traditional Chinese medicine (TCM) concept of "fire-heat syndrome". Huanglian Jiedu Decoction (HLJD), a TCM formula known for clearing heat and purging fire, has shown therapeutic effects on IS, potentially by regulating pyroptosis. Study design: Eight-week-old male mice were divided into six groups: sham operation, model, positive drug, and low-, medium-, and high-dose HLJD groups. After a week of adaptive feeding, mice received respective treatments for five days, followed by modeling on the sixth day, with samples collected 23 h post-perfusion. Analyses included multi-omics, physiology, histopathology, virtual drug screening, target affinity assessment, and molecular biology techniques to measure relevant indicators. Results: HLJD effectively mitigated IS-related damage, maintaining neurological function, reducing ischemic levels, protecting cellular morphology, inhibiting neuronal apoptosis, and preserving blood-brain barrier integrity. Bioinformatics of high-throughput omics data revealed significant activation of pyroptosis and related inflammatory pathways in IS. ScRNA-seq identified neutrophils, macrophages, and microglia as key pyroptotic cell types, suggesting potential therapeutic targets. Network pharmacology and molecular docking identified NLRP3 as a critical target, with 6819 ligand-receptor docking results. SPR molecular fishing, LC-MS, molecular dynamics, and affinity measurements identified small molecules with high affinity for NLRP3. Molecular biology techniques confirmed that HLJD regulates pyroptosis via the classical inflammasome signaling pathway and modulates the inflammatory microenvironment. Conclusions: Following IS, pyroptosis in myeloid cells triggers an inflammatory cascade, leading to neural damage. HLJD may inhibit NLRP3 activity, reducing pyroptosis and associated inflammation, and ultimately mitigating damage.
Keywords: HLJD; drug target relationship; ischemic stroke; multiomics; myeloid cell; pyroptosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Tong-Qiao-Huo-Xue Decoction mitigates post-stroke inflammatory response via suppression of the FIB-NLRP3 signaling pathway.Metab Brain Dis. 2025 May 17;40(5):206. doi: 10.1007/s11011-025-01633-7. Metab Brain Dis. 2025. PMID: 40381107
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
14,15-EET Maintains Mitochondrial Homeostasis to Inhibit Neuronal Pyroptosis After Ischemic Stroke.Stroke. 2025 Jul;56(7):1883-1896. doi: 10.1161/STROKEAHA.124.049143. Epub 2025 Apr 16. Stroke. 2025. PMID: 40235438
References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Poh L., Kang S.-W., Baik S.-H., Ng G.Y.Q., She D.T., Balaganapathy P., Dheen S.T., Magnus T., Gelderblom M., Sobey C.G., et al. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain. Behav. Immun. 2019;75:34–47. doi: 10.1016/j.bbi.2018.09.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources

