Biotechnological Utilization of Amazonian Fruit: Development of Active Nanocomposites from Bacterial Cellulose and Silver Nanoparticles Based on Astrocaryum aculeatum (Tucumã) Extract
- PMID: 40573196
- PMCID: PMC12195901
- DOI: 10.3390/ph18060799
Biotechnological Utilization of Amazonian Fruit: Development of Active Nanocomposites from Bacterial Cellulose and Silver Nanoparticles Based on Astrocaryum aculeatum (Tucumã) Extract
Abstract
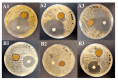
Background/Objectives: The rise of bacterial resistance and the search for alternative, biocompatible antimicrobial materials have driven interest in natural-based nanocomposites. In this context, silver nanoparticles (AgNPs) have shown broad-spectrum antibacterial activity, and bacterial cellulose (BC) is widely recognized for its high purity, hydrophilicity, and biocompatibility. This study aimed to develop a bio-based BC-AgNP nanocomposite via green synthesis using Astrocaryum aculeatum (tucumã) extract and assess its antimicrobial performance for wound dressing applications. Methods: BC was biosynthesized via green tea fermentation (20 g/L tea and 100 g/L sugar) and purified prior to use. AgNPs were obtained by reacting aqueous tucumã extract with silver nitrate (0.1 mmol/L) at pH (9) and temperature (40 °C). BC membranes were immersed in the AgNPs dispersion for 7 days to form the nanocomposite. Characterization was performed using UV-Vis, DLS, TEM, SEM-EDS, FTIR, XRD, ICP-OES, and swelling analysis. Antibacterial activity was evaluated using the disk diffusion method against Staphylococcus aureus and Escherichia coli (ATCC 6538 and 4388). Results: The UV-Vis spectra revealed a gradual decrease in the surface plasmon resonance (SPR) band over 7 days of incubation with BC, indicating progressive incorporation of AgNPs into the membrane. ICP analysis confirmed silver incorporation in the BC membrane at 0.00215 mg/mL, corresponding to 15.5% of the initial silver content. Antimicrobial assays showed inhibition zones of 6.5 ± 0.5 mm for S. aureus and 4.3 ± 0.3 mm for E. coli. Conclusions: These findings validate the successful formation and antimicrobial performance of the BC-AgNP nanocomposite, supporting its potential use in wound care applications.
Keywords: bacterial cellulose; green chemistry; silver nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Kumar Y. Antimicrobial Resistance: A Global Threat. BoD–Books on Demand; Norderstedt, Germany: 2019.
LinkOut - more resources
Full Text Sources

