Unveiling Palmitoyl Thymidine Derivatives as Antimicrobial/Antiviral Inhibitors: Synthesis, Molecular Docking, Dynamic Simulations, ADMET, and Assessment of Protein-Ligand Interactions
- PMID: 40573203
- PMCID: PMC12196243
- DOI: 10.3390/ph18060806
Unveiling Palmitoyl Thymidine Derivatives as Antimicrobial/Antiviral Inhibitors: Synthesis, Molecular Docking, Dynamic Simulations, ADMET, and Assessment of Protein-Ligand Interactions
Abstract
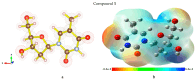
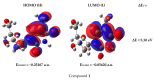
Background/Objectives: Nucleoside precursors and derivatives play pivotal roles in the development of antimicrobial and antiviral therapeutics. The 2022 global outbreak of monkeypox (Mpox) across more than 100 nonendemic countries underscores the urgent need for novel antiviral agents. This study aimed to synthesize and evaluate a series of 5'-O-(palmitoyl) derivatives (compounds 2-6), incorporating various aliphatic and aromatic acyl groups, for their potential antimicrobial activities. Methods: The structures of the synthesized derivatives were confirmed through physicochemical, elemental, and spectroscopic techniques. In vitro antibacterial efficacy was assessed, including minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determinations for the most active compounds (4 and 5). The antifungal activity was evaluated based on mycelial growth inhibition. Density functional theory (DFT) calculations were employed to investigate the electronic and structural properties, including the global reactivity, frontier molecular orbital (FMO), natural bond orbital (NBO), and molecular electrostatic potential (MEP). Molecular docking studies were conducted against the monkeypox virus and the Marburg virus. The top-performing compounds (3, 5, and 6) were further evaluated via 200 ns molecular dynamics (MD) simulations. ADMET predictions were performed to assess drug-likeness and pharmacokinetic properties. Results: Compounds 4 and 5 demonstrated remarkable antibacterial activity compared with the precursor molecule, while most derivatives inhibited fungal mycelial growth by up to 79%. Structure-activity relationship (SAR) analysis highlighted the enhanced antibacterial/antifungal efficacy with CH3(CH2)10CO- and CH3(CH2)12CO-acyl chains. In silico docking revealed that compounds 3, 5, and 6 had higher binding affinities than the other derivatives. MD simulations confirmed the stability of the protein-ligand complexes. ADMET analyses revealed favorable drug-like profiles for all the lead compounds. Conclusions: The synthesized compounds 3, 5, and 6 exhibit promising antimicrobial and antiviral activities. Supported by both in vitro assays and comprehensive in silico analyses, these derivatives have emerged as potential candidates for the development of novel therapeutics against bacterial, fungal, and viral infections, including monkeypox and Marburg viruses.
Keywords: DFT; Marburg virus; microorganism; molecular dynamics simulation; monkeypox; thymidine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





















Similar articles
-
Synthesis, Antimicrobial, Molecular Docking Against Bacterial and Fungal Proteins and In Silico Studies of Glucopyranoside Derivatives as Potent Antimicrobial Agents.Chem Biodivers. 2024 Sep;21(9):e202400932. doi: 10.1002/cbdv.202400932. Epub 2024 Aug 16. Chem Biodivers. 2024. PMID: 38949892
-
Discovery of Severe Acute Respiratory Syndrome Coronavirus 2 Main Protease Inhibitors through Rational Design of Novel Fluorinated 1,3,4-oxadiazole Amide Derivatives: An In-Silico Study.Chem Biodivers. 2025 Jun;22(6):e202403179. doi: 10.1002/cbdv.202403179. Epub 2025 Feb 14. Chem Biodivers. 2025. PMID: 39853882
-
Antidiabetic Potential of Synthesized Ferrocenylmethylaniline Derivatives: Insights From In Vitro Studies, Molecular Docking, ADMET, DFT Calculations, and Molecular Dynamics Simulation.Biotechnol Appl Biochem. 2025 Jun 18. doi: 10.1002/bab.70014. Online ahead of print. Biotechnol Appl Biochem. 2025. PMID: 40534200
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Staszewski S., Morales-Ramirez J., Tashima K.T., Rachlis A., Skiest D., Stanford J., Stryker R., Johnson P., Labriola D.F., Farina D. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N. Engl. J. Med. 1999;341:1865–1873. doi: 10.1056/NEJM199912163412501. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

