Epithelial Cell Dysfunction in Pulmonary Fibrosis: Mechanisms, Interactions, and Emerging Therapeutic Targets
- PMID: 40573209
- PMCID: PMC12195837
- DOI: 10.3390/ph18060812
Epithelial Cell Dysfunction in Pulmonary Fibrosis: Mechanisms, Interactions, and Emerging Therapeutic Targets
Abstract
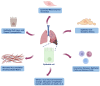
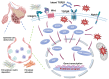
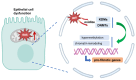
Pulmonary fibrosis (PF) is a progressive and fatal interstitial lung disease characterized by chronic epithelial injury and excessive deposition of extracellular matrix (ECM) driven by dysregulated repair. Increasing evidence has shown that epithelial cell dysfunction plays a key role in PF, involving epithelial-mesenchymal transition (EMT), chronic oxidative stress, disruption of epithelial-immune interactions, and promoting pathological remodeling. Single-cell analyses have identified functionally distinct subpopulations of type 2 alveolar (AT2) cells with pro-fibrotic potential. Epithelial cells exhibit metabolic and epigenetic alterations during PF, which provide new approaches for therapeutic targets. This review summarizes the molecular mechanisms driving epithelial dysfunction in fibrosis progression, with a focus on key regulatory pathways, including transforming growth factor-beta (TGF-β), Wnt, and Notch signaling pathways, as well as miRNA-mediated networks. We also explored emerging epithelial-targeted therapies, ranging from FDA-approved agents (pirfenidone, nintedanib) to experimental inhibitors targeting Galectin-3 and Wnt/β-catenin, providing insights into precision anti-fibrosis strategies for clinical translation.
Keywords: antifibrosis strategies; cell heterogeneity; epithelial dysfunction; epithelial–mesenchymal transition (EMT); pulmonary fibrosis (PF).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

