Impact of Antiglaucoma Drug Number and Class on Corneal Epithelial Thickness Measured by OCT
- PMID: 40573263
- PMCID: PMC12195675
- DOI: 10.3390/ph18060868
Impact of Antiglaucoma Drug Number and Class on Corneal Epithelial Thickness Measured by OCT
Abstract
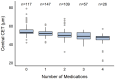
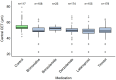
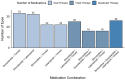
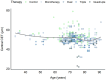
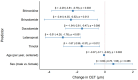
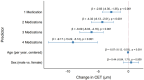
Background/Objectives: The corneal epithelium plays a vital role in maintaining corneal transparency and ocular surface integrity. Chronic topical use of antiglaucoma medications may induce epithelial changes, especially with the concurrent use of multiple agents. This study aimed to evaluate the association between the number and class of antiglaucoma medications and central corneal epithelial thickness (CET), measured using a spectral-domain optical coherence tomography (SD-OCT) device. Methods: This cross-sectional study included 456 eyes from 242 adults (median age 72 years), grouped by the number of antiglaucoma agents used (0-4 medications). All pharmacologically treated participants had received the same regimen for ≥6 months. CET was measured using SD-OCT (SOLIX, Optovue). Generalized estimating equations (GEEs) accounted for inter-eye correlation. Two models were constructed: one evaluating specific medication effects and another assessing CET reduction per additional drug used. Age and sex were included as covariates. Results: CET progressively decreased with the number of medications, ranging from 53 µm in controls to 48 µm with quadruple therapy. Multivariable GEE analysis confirmed a cumulative thinning effect, with each additional medication associated with further CET reduction (β = -2.83 to -9.17 µm, p < 0.001). Latanoprost exerted the most pronounced single-drug effect (β = -3.01 µm, p < 0.001). Age was a modest negative predictor, while sex showed no significant effect. Conclusions: The cumulative number and specific class of antiglaucoma medications have a significant impact on corneal epithelial thickness. These results emphasize the need for vigilant ocular surface evaluation in patients on multi-drug regimens and propose CET as a surrogate marker for the burden of topical therapy.
Keywords: antiglaucoma therapy; benzalkonium chloride; corneal epithelial thickness; glaucoma; latanoprost; ocular surface disease; optical coherence tomography; polypharmacy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty.Cochrane Database Syst Rev. 2017 Feb 23;2(2):CD010746. doi: 10.1002/14651858.CD010746.pub2. Cochrane Database Syst Rev. 2017. PMID: 28231380 Free PMC article.
-
Interventions for recurrent corneal erosions.Cochrane Database Syst Rev. 2018 Jul 9;7(7):CD001861. doi: 10.1002/14651858.CD001861.pub4. Cochrane Database Syst Rev. 2018. PMID: 29985545 Free PMC article.
-
Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD008081. doi: 10.1002/14651858.CD008081.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Jan 07;1:CD008081. doi: 10.1002/14651858.CD008081.pub3. PMID: 21735421 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Ludwig P., Lopez M., Sevensma K. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2023. Anatomy, Head and Neck, Eye Cornea. - PubMed
-
- Nishida T., Saika S., Morishige N. Cornea and Sclera: Anatomy and Physiology. Cornea. 2021;1:1–22.
-
- Ramos M., Attar M., Stern M., Brassard J., Kim A., Matsumoto S., Vangyi C. Safety Pharmacology in Ocular Drug Development. Elsevier; Amsterdam, The Netherlands: 2017. Safety Evaluation of Ocular Drugs.
-
- Collin J., Queen R., Zerti D., Bojic S., Dorgau B., Moyse N., Molina M.M., Yang C., Dey S., Reynolds G., et al. A Single Cell Atlas of Human Cornea That Defines Its Development, Limbal Progenitor Cells and Their Interactions with the Immune Cells. Ocul. Surf. 2021;21:279–298. doi: 10.1016/j.jtos.2021.03.010. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

