Carbon Dots Extracted from the Plant Gardenia jasminoides Ameliorates Ischemia-Reperfusion Injury
- PMID: 40573265
- PMCID: PMC12195920
- DOI: 10.3390/ph18060870
Carbon Dots Extracted from the Plant Gardenia jasminoides Ameliorates Ischemia-Reperfusion Injury
Abstract
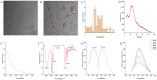
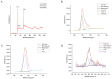
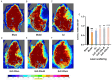
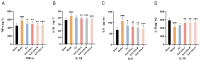
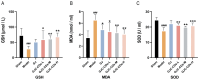
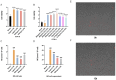
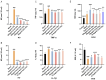
Background: Ischemic stroke (IS) is probably the most important acute serious illness, where interdisciplinary approach is essential to offer the best chance for survival and functional recovery of patients. Carbon dots (CDs) with multifaceted advantages have provided hope for development brand-new nanodrug for treating thorny diseases. Methods: This study developed a green and environmentally responsible calcination method to prepare novel Gardenia jasminoides Carbonisata (GJC-CDs) as promising drug for ischemic stroke treatment. Results: In this work, we isolated and characterized for the first time a novel carbon dots (GJC-CDs) from the natural plant G. jasminoides. Results displayed that green GJC-based CDs with tiny sizes and abundant functional groups exhibited solubility, which may be beneficial for its settled biological activity. The neuroprotective effect of carbon dots from G. jasminoides were evaluated using the classical middle cerebral artery occlusion (MCAO) model. Assessing the infarct volume content of the ischemic cerebral hemisphere and determining the serum tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), reduced glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) levels of the mice in each group, it was evident that pre-administration of the drug by GJC-CDs significantly reduced the infarct volume as well as attenuated inflammatory responses and excessive oxidative stress in MCAO mice. Furthermore, in vitro cellular experiments demonstrated that GJC-CDs have good biosafety and anti-inflammatory and antioxidant capacity. Conclusions: Overall, GJC-CDs performs neuroprotective effect on cerebral ischemia and reperfusion injury, which not only provides evidence for further broadening the biological application of acute ischemic stroke but also offers novel strategy for the application of nanomedicine to treat acute diseases.
Keywords: Gardenia jasminoides; carbon dots; inflammation; ischemic stroke; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Thrombolysis for acute ischaemic stroke.Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. PMID: 12917889 Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Beharry J., Yogendrakumar V., Barros G.W.F., Davis S.M., Norrving B., Figtree G.A., Donnan G., von Euler M., Eriksson M. Mortality in ischaemic stroke patients without standard modifiable risk factors: An analysis of the Riksstroke registry. Eur. Stroke J. 2025:23969873241309516. doi: 10.1177/23969873241309516. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

