Thermosensitive Mucoadhesive Intranasal In Situ Gel of Risperidone for Nose-to-Brain Targeting: Physiochemical and Pharmacokinetics Study
- PMID: 40573266
- PMCID: PMC12196403
- DOI: 10.3390/ph18060871
Thermosensitive Mucoadhesive Intranasal In Situ Gel of Risperidone for Nose-to-Brain Targeting: Physiochemical and Pharmacokinetics Study
Abstract
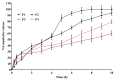
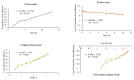
Background/Objectives: Non-invasive central nervous system (CNS) therapies are limited by complex mechanisms and the blood-brain barrier, but nasal delivery offers a promising alternative. The study planned to develop a non-invasive in situ intranasal mucoadhesive thermosensitive gel to deliver CNS-active risperidone via nose-to-brain targeting. Risperidone, a second-generation antipsychotic, has shown efficacy in managing both psychotic and mood-related symptoms. The mucoadhesive gel formulations help to prolong the residence time at the nasal absorption site, thereby facilitating the uptake of the drug. Methods: The poloxamer 407 (18.0% w/v), HPMC K100M and K15M (0.3-0.5% w/v), and benzalkonium chloride (0.1% v/v) were used as thermosensitive polymers, a mucoadhesive agent, and a preservative, respectively, for the development of in situ thermosensitive gel. The developed formulations were evaluated for various parameters. Results: The pH, gelation temperature, gelation time, and drug content were found to be 6.20 ± 0.026-6.37 ± 0.015, 34.25 ± 1.10-37.50 ± 1.05 °C, 1.65 ± 0.30-2.50 ± 0.55 min, and 95.58 ± 2.37-98.03 ± 1.68%, respectively. Furthermore, the optimized F3 formulation showed satisfactory gelling capacity (9.52 ± 0.513 h) and an acceptable mucoadhesive strength (1110.65 ± 6.87 dyne/cm2). Diffusion of the drug through the egg membrane depended on the formulation's viscosity, and the F3 formulation explained the first-order release kinetics, indicating concentration-dependent drug diffusion with n < 0.45 (0.398) value, indicating the Fickian-diffusion (diffusional case I). The pharmacokinetic study was performed with male Wistar albino rats, and the F3 in situ thermosensitive risperidone gel confirmed significantly (p < 0.05) ~5.4 times higher brain AUC0-∞ when administered intranasally compared to the oral solution. Conclusions: Based on physicochemical, in vitro, and in vivo parameters, it can be concluded that in situ thermosensitive gel is suitable for administration of risperidone through the nasal route and can enhance patient compliance through ease of application and with less repeated administration.
Keywords: CNS disorders; blood–brain barrier; diffusion; non-invasive; nose-to-brain targeting; thermosensitive in situ gels.
Conflict of interest statement
Sanjay Kumar is employed by Naari Pharma Private Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures






References
-
- Shakeel F., Alanazi F.K., Alsarra I.A., Haq N. Solubility of Antipsychotic Drug Risperidone in Transcutol+ Water Co-Solvent Mixtures at 298.15 to 333.15 K. J. Mol. Liq. 2014;191:68–72. doi: 10.1016/j.molliq.2013.11.026. - DOI
-
- Shukla D., Chakraborty S., Singh S., Mishra B. Preparation and In-Vitro Characterization of Risperidone-Cyclodextrin Inclusion Complexes as a Potential Injectable Product. DARU. 2009;17:226–235.
-
- Wen P., Ren C. Research Progress on Intranasal Treatment for Parkinson’s Disease. Neuroprotection. 2024;2:79–99. doi: 10.1002/nep3.42. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

