Advancing Regulatory Oversight of Medical Device Trials to Align with Clinical Drug Standards in the European Union
- PMID: 40573269
- PMCID: PMC12195702
- DOI: 10.3390/ph18060876
Advancing Regulatory Oversight of Medical Device Trials to Align with Clinical Drug Standards in the European Union
Abstract
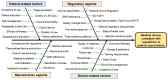
The regulation of clinical trials for medicinal products and medical devices has undergone numerous changes in recent years in the European Union, challenging manufacturers and national regulatory agencies as well. With the introduction of combined drug-device products, the regulatory landscape has been drastically changed to adapt to novel technological advancements and innovations. A comparative analysis has not yet been published highlighting the main differences and common elements of these two medicinal products, which took up almost all of the market in the pharmaceutical sector. Due to stricter regulations in the field of medical devices, the process from application up until post-market surveillance became more difficult, but a correlation between the regulation of drug trials can also be found. The main differences lie in the risk management systems, where, regardless of the background knowledge of a drug, it is always strict and mandatory structured progress, while in the case of medical devices, it is more flexible based on the risk category of the product. Generally, the utilization of e-health opportunities, transparency, and data accessibility have been improved in both fields. Via the adaptation of the mentioned regulation in the EU, the safety of patients and the efficacy of trials have been greatly increased. This manuscript aims to compare the specific regulations of these two types of medicinal products with a brief outlook on the non-EU sector as well.
Keywords: clinical trial; clinical trials regulation; drug development; medical device; medical device regulation; medicinal product; regulatory science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Single-incision sling operations for urinary incontinence in women.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD008709. doi: 10.1002/14651858.CD008709.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Oct 27;10:CD008709. doi: 10.1002/14651858.CD008709.pub4. PMID: 28746980 Free PMC article. Updated.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD002126. doi: 10.1002/14651858.CD002126.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2015 Apr 30;(4):CD002126. doi: 10.1002/14651858.CD002126.pub3. PMID: 16235297 Updated.
References
-
- Asgardoon M.H., Amirzade-Iranaq M.H., Mehri A., Piri S.M., Jalali P., Ghodsi Z., Dehghan H.R., Rahimi-Movaghar V., Salamati P. Adverse Impacts of Imposing International Economic Sanctions on Health. Arch. Iran. Med. 2022;25:182–190. - PubMed
-
- DeGroot L., Pavlovic N., Perrin N., Dy S.M., Szanton S., Gilotra N., Denfeld Q., Miller H., McIlvennan C., Davidson P., et al. The Impact of Unmet Palliative Care Needs and Physical Frailty on Clinical Outcomes: A Prospective Study of Adults with Heart Failure. J. Card. Fail. 2024;30:129. doi: 10.1016/j.cardfail.2023.10.032. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

