Exploring the Pharmacogenetic Landscape: Identification of Clinically Relevant Genotypes by a Nation-Wide Medical Testing Laboratory in Romania
- PMID: 40573293
- PMCID: PMC12195894
- DOI: 10.3390/ph18060898
Exploring the Pharmacogenetic Landscape: Identification of Clinically Relevant Genotypes by a Nation-Wide Medical Testing Laboratory in Romania
Abstract
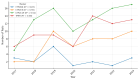
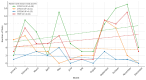
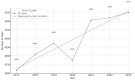
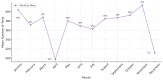
Background: Pharmacogenetic testing aims to assess the existence of a genetic predisposition that could influence the efficacy or safety of pharmacotherapy. The objective of the present study was to offer a descriptive analysis of the results of the pharmacogenetic tests carried out between 2017 and 2023 within the Synevo laboratories, a provider of medical testing with national expansion. Method: To carry out this analysis, data on the following tests offered by the Synevo laboratories were evaluated: CYP2D6, CYP2C9, CYP2C19, TPMT (thiopurine S-methyltransferase), and factor V Leiden. For each type of test, information was collected on the demographics of the patients tested, as well as the genotyping test result. Data were statistically analyzed and interpreted. Results: In total, 31.453 pharmacogenetic tests were performed in the considered time interval. Most patients for whom pharmacogenetic testing was performed were women (80%), and as for the age range, patients between 31 and 40 years old (45%) and those between 19 and 30 years old (38%) predominated. In the evaluated sample, genetic variants associated with a potential risk of influencing pharmacotherapy could be identified in a proportion of 56% for the CYP2D6 gene, 41% for the CYP2C9 gene, 52% for the CYP2C19 gene, 12% for the TPMT gene, and 16% for factor V Leiden. Conclusions: Pharmacogenetic tests can provide useful information to clinicians in order to personalize pharmacotherapy. Although the interest of medical professionals in these tests is increased, there are currently several impediments to the prescription and routine clinical implementation of pharmacogenetic testing.
Keywords: CYP2C19; CYP2C9; CYP2D6; Eastern Europe; Romania genotyping; TPMT; factor V Leiden; personalized medicine; pharmacogenetics.
Conflict of interest statement
The author C.A. is employed by the company Synevo Laboratories. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
-
- Dunnenberger H.M., Crews K.R., Hoffman J.M., Caudle K.E., Broeckel U., Howard S.C., Hunkler R.J., Klein T.E., Evans W.E., Relling M.V. Preemptive Clinical Pharmacogenetics Implementation: Current Programs in Five US Medical Centers. Annu. Rev. Pharmacol. Toxicol. 2015;55:89–106. doi: 10.1146/annurev-pharmtox-010814-124835. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

