Long-Term Outcomes of Ledipasvir/Sofosbuvir Treatment in Hepatitis C: Viral Suppression, Hepatocellular Carcinoma, and Mortality in Mongolia
- PMID: 40573334
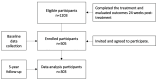
- PMCID: PMC12197668
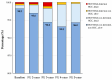
- DOI: 10.3390/v17060743
Long-Term Outcomes of Ledipasvir/Sofosbuvir Treatment in Hepatitis C: Viral Suppression, Hepatocellular Carcinoma, and Mortality in Mongolia
Abstract
(1) Background: Hepatitis C virus (HCV) infection poses a significant health burden, particularly in Mongolia, where the HCV prevalence is notably high. This study evaluates the long-term outcomes of HCV treatment with ledipasvir/sofosbuvir, focusing on mortality, viral relapse, and hepatocellular carcinoma (HCC) development. (2) Methods: This prospective, longitudinal cohort study initially enrolled patients with chronic HCV in Mongolia between 2016 and 2017, focusing on those who completed the five-year follow-up (n = 303). The study measured long-term mortality, HCC development, and viral relapse, employing non-invasive methods to assess liver fibrosis and liver function. (3) Results: At the outset, 98.2% of the patients achieved undetectable HCV RNA levels. Over five years, 6.27% experienced viral relapse and 3.30% developed hepatocellular carcinoma (HCC), with a mortality rate of 5.94%. In a multivariable analysis, the significant predictors for HCC occurrence included age (OR = 1.081, 95% CI = 1.021-1.145), liver cirrhosis (OR = 5.866, 95% CI = 1.672-22.577), and GGT level (OR = 1.011, 95% CI = 1.004-1.018). The independent predictors of mortality included age (OR = 1.083, 95% CI = 1.024-1.147), liver cirrhosis (OR = 6.529, 95% CI = 1.913-22.281), and GGT (OR = 1.011, 95% CI = 1.004-1.017). (4) Conclusions: This study demonstrates that ledipasvir/sofosbuvir effectively suppresses HCV initially and maintains low viral relapse rates over the long term. However, it emphasizes the need for continued management to reduce the long-term risk of HCC and mortality, especially in patients with severe liver fibrosis or cirrhosis.
Keywords: antiviral therapy; chronic liver disease; liver cirrhosis; viral suppression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Baatarkhuu O., Gerelchimeg T., Munkh-Orshikh D., Batsukh B., Sarangua G., Amarsanaa J. Epidemiology, Genotype Distribution, Prognosis, Control, and Management of Viral Hepatitis B, C, D, and Hepatocellular Carcinoma in Mongolia. Euroasian J. Hepato-Gastroenterol. 2018;8:57–62. doi: 10.5005/jp-journals-10018-1260. - DOI - PMC - PubMed
-
- Dambadarjaa D., Radnaa O., Khuyag S.O., Shagdarsuren O.E., Enkhbayar U., Mukhtar Y., Tsogzolbaatar E.O., Nyam G., Shaarii S., Singh P., et al. Hepatitis B, C, and D Virus Infection among Population Aged 10–64 Years in Mongolia: Baseline Survey Data of a Nationwide Cancer Cohort Study. Vaccines. 2022;10:1928. doi: 10.3390/vaccines10111928. - DOI - PMC - PubMed
-
- Baatarkhuu O., Kim D.Y., Ahn S.H., Nymadawa P., Dahgwahdorj Y., Shagdarsuren M., Han K.H. Prevalence and genotype distribution of hepatitis C virus among apparently healthy individuals in Mongolia: A population-based nationwide study. Liver Int. 2008;28:1389–1395. doi: 10.1111/j.1478-3231.2008.01820.x. - DOI - PubMed
-
- The CDA Foundation . Hepatitis C—[Mongolia] CDA Foundation; Lafayette, CO, USA: 2017. [(accessed on 23 February 2025)]. Available online: https://cdafound.org/polaris-countries-database/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous