Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model
- PMID: 40573353
- PMCID: PMC12197658
- DOI: 10.3390/v17060762
Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model
Abstract
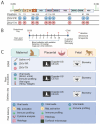
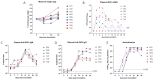
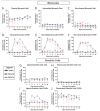
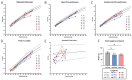
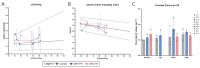
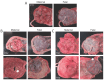
Zika virus (ZIKV) infection during pregnancy can cause a broad range of neurological birth defects, collectively named Congenital Zika Syndrome (CZS). We have previously shown that infection with the Puerto Rican isolate PRVABC59 (ZIKV-PR) results in abnormal oxygen transport in the placenta due to villous damage and uterine vasculitis in a nonhuman primate model. To investigate whether this type of damage occurs with endemically circulating strains in Thailand, we investigated a CZS case isolate, MU1-2017 (ZIKV-TH), in pregnant rhesus macaques. Pregnant animals (n = 3 per group) were infected subcutaneously with either ZIKV-PR or ZIKV-TH at ~50 days gestation (GD) and monitored for 40 days post-infection (GD90). Similar courses of viremia and immune activation were observed for both viruses when compared to uninfected controls. In addition, both viruses induced changes to the placental architecture, including spiral artery remodeling and the development of infarctions. Similar levels of viral RNA were detected at necropsy in maternal and fetal tissues. Overall, our results show that the ZIKV-TH strain MU1-2017 behaves similarly to the ZIKV-PR strain, and, importantly, provide evidence of in-utero infection with an additional contemporary strain of ZIKV.
Keywords: Thailand; Zika virus; congenital Zika syndrome; immunology; non-human primate; placenta.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

