Case Series of Adverse Pregnancy Outcomes Associated with Oropouche Virus Infection
- PMID: 40573407
- PMCID: PMC12197463
- DOI: 10.3390/v17060816
Case Series of Adverse Pregnancy Outcomes Associated with Oropouche Virus Infection
Abstract
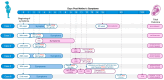
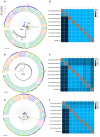
The Oropouche virus (OROV) is an arbovirus (Peribunyaviridae: Orthobunyavirus) that traditionally causes febrile outbreaks in Latin America's Amazon region. Previously, OROV was not associated with severe pregnancy outcomes. During the 2022-2024 outbreak in Brazil, OROV expanded geographically, revealing links to adverse pregnancy outcomes. This study describes six cases with varied fetal outcomes, including miscarriage, antepartum, intrauterine fetal demise (IFD), and normal development, correlating with maternal symptoms but not symptom severity. Vertical transmission was confirmed by detecting OROV through RT-qPCR, ELISA, and immunohistochemistry in fetal tissues. Genome sequencing from an IFD case identified a novel reassortment pattern reported in the 2022-2024 outbreak. Severe encephalomalacia, meningoencephalitis, vascular compromise, and multi-organ damage were evident, underscoring the significant risk OROV poses to fetal development and emphasizing the need for further investigation.
Keywords: fetal outcomes; oropouche virus; vertical transmission.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Chiang J.O., Azevedo R.S., Justino M.C.A., Matos H.J., Cabeça H.L.S., Silva S.P., Henriques D.F., Silva E.V., Andrade G.S., Vasconcelos P.F., et al. Neurological disease caused by Oropouche virus in northern Brazil: Should it be included in the scope of clinical neurological diseases? J. Neurovirol. 2021;27:626–630. doi: 10.1007/s13365-021-00987-9. - DOI - PMC - PubMed
-
- Pinheiro F.P., Freitas A.G., Ohana R.B., Rosa B.A., Travassos da Rosa A.P.A., Juvenal S.L. Meningitis associated with Oropouche virus infections. Rev. Inst. Med. Trop. São Paulo. 1982;24:246–251. - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

