Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity
- PMID: 40573778
- PMCID: PMC12197095
- DOI: 10.3390/plants14121785
Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity
Abstract
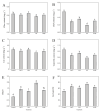
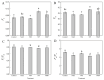
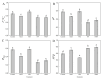
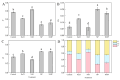
Salt stress severely impairs photosynthesis and development in tomato seedlings. This study investigated the regulatory role of exogenous melatonin (MT) on photosynthetic performance under salt stress by determining chlorophyll content, chlorophyll a fluorescence parameters, Calvin cycle enzyme activities, and related gene expression. Results showed that salt stress significantly reduced chlorophyll content and impaired photosystem II (PSII) functionality, as evidenced by the increased minimum fluorescence (Fo) and decreased maximum quantum efficiency of PSII (Fv/Fm) and effective PSII quantum yield (ΦPSII). MT application mitigated these negative effects, as reflected by higher Fv/Fm, increased chlorophyll content, and lower non-photochemical quenching (NPQ). In addition, MT-treated plants exhibited improved PSII electron transport and more efficient use of absorbed light energy, as shown by elevated ΦPSII and qP values. These changes suggest improved PSII functional stability and reduced excess thermal energy dissipation. Furthermore, MT significantly enhanced both the activity and expression of key enzymes involved in the Calvin cycle, including ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), Rubisco activase (RCA), phosphoglycerate kinase (PGK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), fructose-1,6-bisphosphatase (FBPase), fructose-bisphosphate aldolase (FBA), transketolase (TK), and sedoheptulose-1,7-bisphosphatase (SBPase), thereby promoting carbon fixation and ribulose-1,5-bisphosphate (RuBP) regeneration under salt stress. Conversely, inhibition of endogenous MT synthesis by p-CPA exacerbated salt stress damage, further confirming MT's crucial role in salt tolerance. These findings demonstrate that exogenous MT enhances salt tolerance in tomato seedlings by simultaneously improving photosynthetic electron transport efficiency and upregulating the activity and gene expression of key Calvin cycle enzymes, thereby promoting the coordination between light reactions and carbon fixation processes. This study provides valuable insights into the comprehensive regulatory role of MT in maintaining photosynthetic performance under saline conditions.
Keywords: Calvin cycle; chlorophyll a fluorescence; melatonin; salt stress; tomato seedlings.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Protective mechanisms of exogenous melatonin on chlorophyll metabolism and photosynthesis in tomato seedlings under heat stress.Front Plant Sci. 2025 Feb 4;16:1519950. doi: 10.3389/fpls.2025.1519950. eCollection 2025. Front Plant Sci. 2025. PMID: 39967814 Free PMC article.
-
Silicon-induced photosynthetic adaptations in common buckwheat under salt stress revealed by prompt chlorophyll a fluorescence analysis.Sci Rep. 2025 Jun 2;15(1):19343. doi: 10.1038/s41598-025-04159-1. Sci Rep. 2025. PMID: 40456900 Free PMC article.
-
Effects of cadmium (Cd) on photosynthetic characteristics and chlorophyll fluorescence parameters in the ornamental Plant Salvia splendens Ker-Gawl.Physiol Mol Biol Plants. 2025 Mar;31(3):507-519. doi: 10.1007/s12298-025-01584-4. Epub 2025 Apr 6. Physiol Mol Biol Plants. 2025. PMID: 40256272
-
Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review.Int J Mol Sci. 2023 Nov 9;24(22):16123. doi: 10.3390/ijms242216123. Int J Mol Sci. 2023. PMID: 38003311 Free PMC article.
-
Regulation of Calvin-Benson cycle enzymes under high temperature stress.aBIOTECH. 2022 Jan 24;3(1):65-77. doi: 10.1007/s42994-022-00068-3. eCollection 2022 Mar. aBIOTECH. 2022. PMID: 36311539 Free PMC article. Review.
References
-
- Al-Gaadi K.A., Tola E., Madugundu R., Zeyada A.M., Alameen A.A., Edrris M.K., Edrees H.F., Mahjoop O. Response of Leaf Photosynthesis, Chlorophyll Content and Yield of Hydroponic Tomatoes to Different Water Salinity Levels. PLoS ONE. 2024;19:e0293098. doi: 10.1371/journal.pone.0293098. - DOI - PMC - PubMed
-
- Borbély P., Iqbal N., Czékus Z., Tari I., Poór P. Exogenous Sodium Nitroprusside Alleviates Salt-Induced Changes in Photosynthesis of Greenhouse Tomato Plants by Leaf Age-Dependent Manner. J. Plant Growth Regul. 2025:1–13. doi: 10.1007/s00344-025-11707-6. - DOI
-
- Boorboori M.R., Li J. The Effect of Salinity Stress on Tomato Defense Mechanisms and Exogenous Application of Salicylic Acid, Abscisic Acid, and Melatonin to Reduce Salinity Stress. Soil Sci. Plant Nutr. 2025;71:93–110. doi: 10.1080/00380768.2024.2405834. - DOI
Grants and funding
- Qian Jiaoji [2024]232/the Growth of Young Scientific and Techno-logical Talents of Guizhou Educational Commission
- grant No. BS20240218/the Specialized Fund for the Doctoral of Kaili University
- MSGZS-SJ-2024002/Provincial famous teacher Yang Qin studio
- No. Qianjiaoji [2022] 053/the Key Laboratory of the Department of Education of Guizhou Province
LinkOut - more resources
Full Text Sources
Research Materials

