Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological-Transcriptomic Study
- PMID: 40573789
- PMCID: PMC12196763
- DOI: 10.3390/plants14121802
Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological-Transcriptomic Study
Abstract
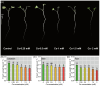
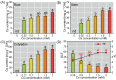
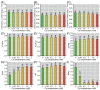
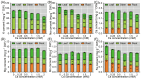
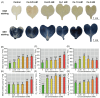
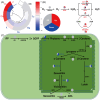
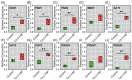
The present study systematically investigated the cesium (Cs) enrichment characteristics and physiological responses to Cs exposure in radish (Raphanus sativus L.) seedlings under hydroponic conditions through integrated physiological, biochemical, and transcriptome analyses. The results showed that the Cs content in radish roots, stems, and cotyledons increased progressively with rising Cs concentrations (0.25-2 mM), and Cs mainly accumulated in the cotyledon. The transfer factor (TF) increased by 63.29% (TF = 3.87) as the Cs concentration increased from 0.25 to 2 mM, while the biological concentration factor (BCF) decreased by 72.56% (BCF = 14.87). Severe growth inhibition was observed at 2 mM Cs stress, with biomass reduction reaching 29.73%. The carotenoid content decreased by 11.92%; however, the total chlorophyll content did not change significantly, and the photosynthesis of radish was not affected. In addition, Cs exposure disrupted mineral nutrient homeostasis, decreasing potassium (K), sodium (Na), magnesium (Mg), and iron (Fe) content. The superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities, reactive oxygen species (ROS), and malondialdehyde (MDA) content increased under the different Cs treatments, which indicated that Cs exposure induced oxidative stress response in radish seedlings. Transcriptome analysis detected a total of 4326 differentially expressed genes (DEGs), in which altered expression patterns in genes associated with mineral transport, antioxidant systems, and carotenoid biosynthesis pathways in radish under 2 mM Cs treatment were observed. In conclusion, this study comprehensively investigated the physiological and molecular responses of radish to Cs stress, revealing that Cs accumulation exhibited site-specific preference and concentration dependence and induced physiological disturbances, including growth inhibition and photosynthetic pigment metabolism alterations. At the transcription level, Cs activated the enzymatic antioxidant system, related genes, and stress-response pathways. Notably, this study is the first to demonstrate that Cs disrupts plant mineral nutrition homeostasis and inhibits carotenoid biosynthesis. These findings establish a crucial theoretical foundation for utilizing radish in Cs-contaminated phytoremediation strategies.
Keywords: antioxidant; cesium; mineral elements; photosynthesis; reactive oxygen species.
Conflict of interest statement
All authors declare that we have no significant competing financial or professional interests that might have affected the work and presentation described in this manuscript.
Figures



References
-
- Wei F., Chen J., Wu Y., Zheng C. Research on the background values of the Chinese soil environment. Environ. Sci. 1991;12:5–19. doi: 10.13227/j.hjkx.1991.04.005. - DOI
-
- Ashraf M.A., Akib S., Maah M.J., Yusoff I., Balkhair K.S. Cesium-137: Radio-Chemistry, Fate, and Transport, Remediation, and Future Concerns. Crit. Rev. Environ. Sci. Technol. 2014;44:1740–1793. doi: 10.1080/10643389.2013.790753. - DOI
-
- Liu J., Zhao L., Liu Q., Li J., Qiao Z., Sun P., Yang Y. A critical review on soil washing during soil remediation for heavy metals and organic pollutants. Int. J. Environ. Sci. Technol. 2021;19:601–624. doi: 10.1007/s13762-021-03144-1. - DOI
Grants and funding
- No. 17ZB0363/Education Department of Sichuan Province, China
- No. ZZYQ2020008/Experimental Technology and Self-made Instrument Project of Sichuan Normal University, China
- No. KFSY2022002/Open Experiment Project of Sichuan Normal University, China
- No. S202510636101/Innovation and Entrepreneurship Training Program of Sichuan Normal University
LinkOut - more resources
Full Text Sources
Miscellaneous

