Rates of SARS-CoV-2 Breakthrough Infection or Severe COVID-19 and Associated Risk Factors After Primary and Booster Vaccination Against COVID-19 in the Netherlands
- PMID: 40573895
- PMCID: PMC12197751
- DOI: 10.3390/vaccines13060564
Rates of SARS-CoV-2 Breakthrough Infection or Severe COVID-19 and Associated Risk Factors After Primary and Booster Vaccination Against COVID-19 in the Netherlands
Abstract
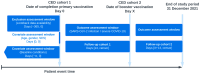
Background: The effectiveness of COVID-19 vaccines appears to decline rapidly over time due to waning immunity and immune evasion by emerging variants of concern, and may be reduced in high-risk populations. We aimed to evaluate the rates of SARS-CoV-2 breakthrough infection or severe COVID-19, both in individuals who had completed their primary COVID-19 vaccination, and in those who had received their first booster vaccination. Specifically, we aimed to evaluate whether persons with certain risk factors, such as age, gender, socioeconomic status (SES), and specified comorbidities have an increased risk of either breakthrough infection or severe COVID-19, compared to those without the respective risk factors. Methods: Data on COVID-19 vaccinations, infections, hospitalizations, and deaths were collected from the PHARMO Data Network, consisting of health records from Dutch residents. Two cohorts were established: (1) all persons who have completed their primary COVID-19 vaccination regimen, and (2) those who have received their first booster vaccination. The outcomes were SARS-CoV-2 breakthrough infection, and severe COVID-19, defined as either hospitalization or death following SARS-CoV-2 infection. Incidence rates of these outcomes were calculated in both cohorts. The adjusted incidence rate ratios of these outcomes in persons with certain risk factors were calculated, using generalized linear models with a Poisson distribution. Results: In 2021, a total of 1,090,567 individuals received either two doses of BNT162b2, AZD1222, or mRNA-1273, or one dose of Ad26.COV2.S and were included in the primary vaccination cohort, of which 344,153 (31.6%) received a booster vaccination. Overall incidence rates of SARS-CoV-2 breakthrough infection and severe COVID-19 after primary vaccination were 29.9 and 3.1 per 1000 person-years, respectively, and after booster vaccination were 256.4 and 2.3, respectively. Male gender, older age, lower SES, history of COVID-19, and recent hospitalization were factors associated with a lower risk of breakthrough infection after primary vaccination, and a higher risk of severe COVID-19. The risk of severe COVID-19 after primary vaccination was increased in persons with several comorbidities, compared to those without, and remained elevated after booster vaccination in persons with diabetes or lung disease. Conclusions: Our study emphasizes the crucial role of boosters in reducing breakthrough infections, particularly in high-risk populations. The varied impact on severe outcomes in individuals with comorbidities underscores the need for ongoing surveillance and tailored vaccination strategies.
Keywords: COVID-19; SARS-CoV-2; comorbidities; coronavirus; risk factors; vaccination; vaccine effectiveness.
Conflict of interest statement
Jesse M. van den Berg and Jetty A. Overbeek are employees and Ron M. C. Herings is scientific director of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for the government, related healthcare authorities, and several pharmaceutical companies. The other authors have declared no competing interests.
Figures


Similar articles
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England.PLoS Med. 2023 Jun 7;20(6):e1004245. doi: 10.1371/journal.pmed.1004245. eCollection 2023 Jun. PLoS Med. 2023. PMID: 37285378 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
-
- Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., AlMukdad S., Yassine H.M., Al-Khatib H.A., Smatti M.K., Tang P., Hasan M.R., Coyle P., et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

