Long-Term Immuno-Response and Risk of Breakthrough Infection After SARS-CoV-2 Vaccination in Kidney Transplantation
- PMID: 40573897
- PMCID: PMC12197762
- DOI: 10.3390/vaccines13060566
Long-Term Immuno-Response and Risk of Breakthrough Infection After SARS-CoV-2 Vaccination in Kidney Transplantation
Abstract
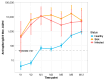
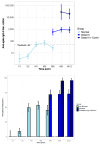
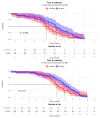
Background: Kidney transplant (KTx) recipients exhibit impaired responses to SARS-CoV-2 vaccination. Correlates of vaccine-induced immunity and risk factors for breakthrough infection are not fully defined. This study evaluated the humoral response trajectories and determinants of breakthrough infection in KTx recipients. Methods: KTx recipients received two doses of the BNT162b2 mRNA vaccine three weeks apart and a booster after six months. Patients were categorized based on pre-vaccination status: previous COVID-19 disease (DIS), asymptomatic SARS-CoV-2 infection (INF), or infection-naïve (NEG). Serum anti-spike antibody titers were assessed at baseline, before the second dose, and at 1, 3, 6, 9, and 12 months. Linear mixed models and survival analyses were performed. Results: Of 326 enrolled patients, 189 with complete time-point data were included in the longitudinal analysis. Antibodies were detectable in 89% of DIS/INF at baseline and 91% before the second dose, but were negligible in NEG. In NEG, the seropositivity increased after vaccination and booster, reaching 78% at 12 months. Age (-5% per year, p < 0.001) and BMI (+10% per unit, p = 0.004) influenced titers; antimetabolites and steroids had strong negative effects (-70%, p = 0.005; -84%, p = 0.001). Breakthrough infections occurred in 104 (31.9%); 40% were asymptomatic, and 2 patients died. An mTOR inhibitor was associated with a reduced infection risk (OR 0.27 [CI: 0.09-0.70], p = 0.009). Higher antibody titers correlated with delayed infection (p = 0.063). Conclusions: In KTx patients, humoral response to SARS-CoV-2 vaccination is limited in infection-naïve patients but improved by booster dosing; the hybrid immunity is more effective. Immunosuppressive regimens influence the immune response, and mTOR inhibitors may protect against breakthrough infection.
Keywords: BNT162b2; COVID; SARS-CoV-2; hybrid immunity; immune response; infection risk; kidney transplant; mRNA vaccine.
Conflict of interest statement
V.B. declares that they have served as a consultant, advisory board member, invited speaker for Dr. Shaer, Fresenius Kabi, and invited speakers at AstraZeneca meetings. The other authors have no conflicts of interest to declare.
Figures


Similar articles
-
Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England.PLoS Med. 2023 Jun 7;20(6):e1004245. doi: 10.1371/journal.pmed.1004245. eCollection 2023 Jun. PLoS Med. 2023. PMID: 37285378 Free PMC article.
-
Rates of SARS-CoV-2 Breakthrough Infection or Severe COVID-19 and Associated Risk Factors After Primary and Booster Vaccination Against COVID-19 in the Netherlands.Vaccines (Basel). 2025 May 26;13(6):564. doi: 10.3390/vaccines13060564. Vaccines (Basel). 2025. PMID: 40573895 Free PMC article.
-
Efficacy and safety of booster vaccination against SARS-CoV-2 in dialysis and renal transplant patients: systematic review and meta-analysis.Int Urol Nephrol. 2023 Apr;55(4):791-802. doi: 10.1007/s11255-023-03471-x. Epub 2023 Feb 1. Int Urol Nephrol. 2023. PMID: 36723829 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article.
-
Characteristics of humoral responses to the first coronavirus disease booster vaccine and breakthrough infection in central China: a multicentre, prospective, longitudinal cohort study.Front Immunol. 2025 Jan 7;15:1446751. doi: 10.3389/fimmu.2024.1446751. eCollection 2024. Front Immunol. 2025. PMID: 39840052 Free PMC article.
References
-
- Jacobson I.M., Jaffers G., Dienstag J.L., Tolkoff-Rubin N.E., Cosimi A.B., Delmonico F., Watkins E., Hinkle C., O’rourke S., Russell P.S., et al. Immunogenicity of Hepatitis B Vaccine in Renal Transplant Recipients. Transplantation. 1985;39:393–395. doi: 10.1097/00007890-198504000-00011. - DOI - PubMed
-
- Luxi N., Giovanazzi A., Capuano A., Crisafulli S., Cutroneo P.M., Fantini M.P., Ferrajolo C., Moretti U., Poluzzi E., Raschi E., et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021;44:1247–1269. doi: 10.1007/s40264-021-01131-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

