Nanoparticle-Based mRNA Vaccine Induces Protective Neutralizing Antibodies Against Infectious Bronchitis Virus in In-Vivo Infection
- PMID: 40573899
- PMCID: PMC12197329
- DOI: 10.3390/vaccines13060568
Nanoparticle-Based mRNA Vaccine Induces Protective Neutralizing Antibodies Against Infectious Bronchitis Virus in In-Vivo Infection
Abstract
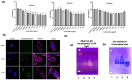
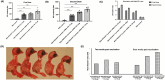
Background: Live attenuated and inactivated virus vaccines are commonly used against infectious bronchitis virus (IBV) in chickens, but they have limitations such as mutation risks and short efficacy. This study explores cationic bovine serum albumin (BSA) polyamine nanoparticles (NPs) for delivering IBV spike protein mRNA, aiming to develop a safer and more effective vaccine. Methods: A BSA-based nanoparticle system was designed with positive surface charges and characterized using dynamic light scattering (DLS), Zetasizer, and transmission electron microscopy (TEM). Its cytotoxicity, cellular uptake, and ability to deliver IBV spike protein mRNA were evaluated in macrophage-like chicken cell lines (HD11), followed by immunogenicity studies in SPF chickens to assess immune responses. Results: The study demonstrated successful binding and transfection efficiency of the in vitro transcription (IVT)-mRNA complexed with the NPs, which was enhanced with chloroquine. Immunogenicity studies in SPF chickens showed a significant increase in antibody titers in chickens vaccinated with the mRNA vaccine compared to the PBS control, indicating an effective immune response against the IBV S protein. Furthermore, the neutralization index doubled after a higher-dose mRNA booster with chloroquine, and PBMCs from immunized chickens exhibited a threefold higher stimulation index than the PBS control. Conclusions: BSA-based NPs effectively deliver IBV spike protein mRNA, enhancing immune responses and offering a promising strategy for a safer, more effective IBV vaccine.
Keywords: infectious bronchitis virus; mRNA vaccine; nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
A novel plant-derived recombinant COBRA infectious bronchitis virus spike protein can elicit a strong immune response in chickens.Vet Res Commun. 2025 May 13;49(4):196. doi: 10.1007/s11259-025-10755-3. Vet Res Commun. 2025. PMID: 40358746
-
Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus.Vaccines (Basel). 2025 Jul 28;13(8):802. doi: 10.3390/vaccines13080802. Vaccines (Basel). 2025. PMID: 40872889 Free PMC article.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Ladman B.S., Pope C.R., Ziegler A.F., Swieczkowski T., Callahan C.J.M., Davison S., Gelb J. Protection of Chickens after Live and Inactivated Virus Vaccination against Challenge with Nephropathogenic Infectious Bronchitis Virus PA/Wolgemuth/98. Avian Dis. 2002;46:938–944. doi: 10.1637/0005-2086(2002)046[0938:POCALA]2.0.CO;2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

