Vaccine Development for Human Pneumoviruses
- PMID: 40573900
- PMCID: PMC12197412
- DOI: 10.3390/vaccines13060569
Vaccine Development for Human Pneumoviruses
Abstract
Background: Pneumoviruses are etiologic agents of respiratory tract infections and a major cause of morbidity and mortality worldwide, particularly affecting young children, the elderly, and individuals with underlying clinical conditions. These viruses are associated with a significant burden, particularly in low- and middle-income countries, where reported deaths attributable to respiratory syncytial virus (RSV) and human metapneumovirus (HMPV) in young children are important. Recent developments have been noted in the prevention of pneumoviral infections.
Method: In this review, we analyzed clinical trials of the approved RSV vaccines, as well as the recent prominent platform technologies used in RSV vaccine research. In addition, we discussed combination vaccines targeting RSV, HMPV, and Human Parainfluenza Virus Type 3 (HPIV3) that have entered clinical trials.
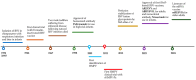
Results: Recent advancements include the approval of three RSV vaccine candidates: AREXVY®(GSK), ABRYSVO®(Pfizer), and mRESVIA®(Moderna). These vaccines are primarily intended for older adults, with ABRYSVO® also capable of providing passive immunization to infants via maternal administration. The review highlights RSV vaccine platform technologies and combination vaccines currently being evaluated in clinical settings.
Conclusions: While significant progress has been made in RSV vaccine development, especially with three approved candidates, the development of vaccines for HMPV remains an unmet medical need. Ongoing research in combination vaccines holds promise for broader protection against multiple respiratory viruses in the future.
Keywords: clinical trial; human metapneumovirus (HMPV); lower respiratory tract infection (LRTI); pneumovirus; respiratory syncytial virus (RSV); vaccine.
Conflict of interest statement
G.B., M.R.C., J.D., and M.-È.H. are cofounders and shareholders of Vaxxel.
Figures

References
-
- Safiri S., Mahmoodpoor A., Kolahi A.A., Nejadghaderi S.A., Sullman M.J.M., Mansournia M.A., Ansarin K., Collins G.S., Kaufman J.S., Abdollahi M. Global burden of lower respiratory infections during the last three decades. Front. Public Health. 2023;10:1028525. doi: 10.3389/fpubh.2022.1028525. - DOI - PMC - PubMed
-
- IHME (Institute for Health Metrics and Evaluation) Global Burden of Disease 2021: Findings from the GBD 2021 Study. IHME; Seattle, WA, USA: 2024.
-
- O’Brien K.L., Baggett H.C., Brooks W.A., Feikin D.R., Hammitt L.L., Higdon M.M., Howie S.R.C., Deloria Knoll M., Kotloff K.L., Levine O.S., et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet. 2019;394:757–779. doi: 10.1016/S0140-6736(19)30721-4. - DOI - PMC - PubMed
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., A Madhi S., Omer S.B., Simões E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

