Construction and Preclinical Evaluation of a Recombinant Attenuated Measles Vaccine Candidate of the H1a Genotype
- PMID: 40573902
- PMCID: PMC12197764
- DOI: 10.3390/vaccines13060571
Construction and Preclinical Evaluation of a Recombinant Attenuated Measles Vaccine Candidate of the H1a Genotype
Abstract
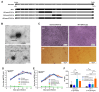
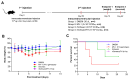
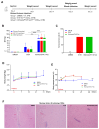
Background: Measles, an acute respiratory infectious disease caused by the measles virus, continues to pose a significant threat to children under five years old worldwide. Despite the availability of effective vaccines, challenges such as insufficient vaccination coverage and antigenic drift contribute to its persistence. Based on a newly isolated wild-type measles virus strain (genotype H1a), designated MVs/Jiangsu.CHN/38.16/1[H1a] (MV-1), this study aims to develop and evaluate a novel recombinant measles virus vaccine candidate designed to enhance immunogenicity and broaden protection against multiple epidemic genotypes. Methods: A recombinant measles virus vaccine candidate, designated rSchwarz/FH(H1a), was developed by incorporating immunogenic genes from the H1a genotype into the backbone of the Schwarz vaccine strain. The genetic stability, safety, and immunogenicity of this vaccine candidate were evaluated in preclinical models. Relevant sample sizes and methodologies were selected to ensure comprehensive assessment of vaccine efficacy against various genotypes (H1a, B3, D8). Results: The rSchwarz/FH(H1a) vaccine candidate demonstrated enhanced immunogenicity, with robust immune responses observed against the targeted genotypes. Additionally, it showed excellent genetic stability and safety profiles, indicating potential for effective use in vaccination programs. Notably, the vaccine provided cross-protection against multiple epidemic genotypes, highlighting its broader application in controlling measles outbreaks. Conclusions: Our findings suggest that the rSchwarz/FH(H1a) vaccine candidate represents a promising advancement in measles vaccine development. It has the potential to strengthen current measles vaccination strategies by providing improved immunogenicity and broader protection against different circulating genotypes. Further clinical trials are warranted to confirm these promising preclinical results.
Keywords: attenuated live; cross-protection; genotype; measles; recombinant virus.
Conflict of interest statement
Authors Lixia Xie, Yajing Zhang, Biao Niu, Hui Wang, Yue Guo, Jinliang Wang, Juncheng Ruan, Guandong Xie, Zhenfang Fu and Dayong Tian were employed by the company Shanghai King-Cell Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Nomenclature for describing the genetic characteristics of wild-type measles viruses (update) Wkly. Epidemiol. Rec. 2001;76:249–251. - PubMed
-
- Update of the nomenclature for describing the genetic characteristics of wild-type measles viruses: New genotypes and reference strains. Wkly. Epidemiol. Rec. 2003;78:229–232. - PubMed
-
- New genotype of measles virus and update on global distribution of measles genotypes. Wkly. Epidemiol. Rec. 2005;80:347–351. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

