Optimized Production of Virus-like Particles in a High-CHO-Cell-Density Transient Gene Expression System for Foot-and-Mouth Disease Vaccine Development
- PMID: 40573912
- PMCID: PMC12197728
- DOI: 10.3390/vaccines13060581
Optimized Production of Virus-like Particles in a High-CHO-Cell-Density Transient Gene Expression System for Foot-and-Mouth Disease Vaccine Development
Abstract
Background/objectives: Foot-and-mouth disease virus (FMDV) poses a continuous threat to livestock health and agricultural economies. Current vaccines require high biosafety standards and are costly to produce. While novel vaccine technologies have been explored, most fail to meet industrial scalability, cost-efficiency, or multiserotype flexibility required for effective FMD control. This study aimed to evaluate the feasibility of using a high-cell density transient gene expression (TGE) system in CHO cells for the production of FMDV virus-like particles (VLPs) as a recombinant vaccine platform.
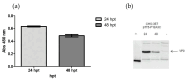
Methods: VLP expression was optimized by adjusting cDNA and polyethyleneimine (PEI) concentrations. Expression yields were compared at 24 and 48 h post-transfection to determine optimal harvest timing. We further tested the system's capacity to express different serotypes and chimeric constructs, incorporating VP1 sequences from various FMDV strains. Immunogenicity was evaluated in swine using VLPs from the A2001 Argentina strain as a model.
Results: Optimal VLP expression was achieved at 24 h post-transfection. Chimeric constructs incorporating heterologous VP1 regions were successfully expressed. Immunized pigs developed protective antibody titers as measured by a virus neutralization test (VNT, log10 titer 1.43) and liquid-phase blocking ELISA (LPBE, titer 2.20) at 28 days post-vaccination (dpv). Titers remained above protective thresholds up to 60 dpv with a single dose. A booster at 28 dpv further elevated titers to levels comparable to those induced by the inactivated vaccine.
Conclusions: Our results demonstrate the feasibility of using CHO cell-based TGE for producing immunogenic FMDV VLPs. This platform shows promise for scalable, cost-effective, and biosafe development of recombinant FMD vaccines.
Keywords: CHO cells; foot-and-mouth disease virus; virus-like particles.
Conflict of interest statement
Author Cintia Sánchez, Romina Scian, Jorge Filippi and Sabrina Beatriz Cardillo were employed by the company Biogénesis Bagó. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- WOAH Foot and Mouth Disease [WWW Document] 2022. [(accessed on 26 January 2025)]. Available online: https://www.woah.org/en/disease/foot-and-mouth-disease/
-
- Anderson I. Foot and Mouth Disease 2001: Lessons to Be Learned Inquiry Report [WWW Document] 2001. [(accessed on 26 January 2025)]. Available online: https://www.jesip.org.uk/uploads/media/incident_reports_and_inquiries/Fo...
-
- Schroeder T.C. Economic Impact of Alternative FMD Emergency Vaccination Strategies in the Midwest United States. J. Agric. Appl. Econ. 2015;47:47. doi: 10.1017/aae.2014.5. - DOI
-
- Grand View Research, Inc. Foot & Mouth Disease Vaccine Market Size Worth $3.0 Billion. [WWW Document] 2017. [(accessed on 26 January 2025)]. Available online: https://www.prnewswire.com/news-releases/foot--mouth-disease-vaccine-mar...
Grants and funding
LinkOut - more resources
Full Text Sources

