Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses
- PMID: 40573951
- PMCID: PMC12197369
- DOI: 10.3390/vaccines13060620
Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses
Abstract
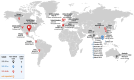
Background/Objectives:H5N1 influenza viruses are spreading worldwide and threaten global public health. Preparedness is necessary to mitigate the worst-case scenario should an H5N1 influenza pandemic occur and justify the development of vaccines against circulating H5N1 viruses of concern.
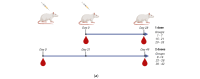
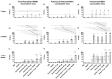
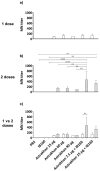
Methods: The production and characterization of egg-based split and inactivated H5Nx of three distinct monovalent antigens from clades 2.3.4.4b, 2.3.2.1c, and 2.3.4 were performed at an industrial scale. These antigens were formulated and their immune responses, when combined or not with IB160 squalene-based oil-in-water emulsion adjuvant in a rat model, were evaluated in a one- or two-dose immunization schedule. IgG antibodies, hemagglutination inhibitions, and microneutralization titers were measured for vaccine-induced immunity and cross-reactivity.
Results: Three monovalent vaccines from clades 2.3.4.4b, 2.3.2.1c, and 2.3.4 were produced at an industrial scale and characterized. The immune responses against the monovalent vaccines showed a clade-specific antibody response and the need to combine with IB160 adjuvant for a required immune response.
Conclusions: Considering the candidate vaccine viruses (CVVs) with the testing potency reagents available and that the antibody response obtained against the CVVs produced was clade-specific, IDCDC RG-71A is the indicated CVV for the predominant currently circulating H5N1 influenza virus of clade 2.3.4.4b and must be combined with adjuvant to induce a higher and efficacious immune response in a two-dose immunization protocol.
Keywords: H5N1 pandemic influenza vaccine; hemagglutination inhibition; immunogenicity; influenza virus; microneutralization; squalene-based emulsion adjuvant.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- EFSA (European Food Safety Authority) ECDC (European Centre for Disease Prevention and Control) EURL (European Union Reference Laboratory for Avian Influenza) Scientific report: Avian influenza overview December 2023–March 2024. EFSA J. 2024;22:8754. doi: 10.2903/j.efsa.2024.8754. - DOI - PMC - PubMed
Grants and funding
- NA/Fundação Butantan
- 2020/07040-1/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 305430/2019-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- 444400/2023-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- 583 309741/2023-8/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
LinkOut - more resources
Full Text Sources

