A Multi-Center Study on Sensitization to Thimerosal in North-Eastern Italy, 1997-2023: Prevalence, Risk Factors, the Role of Occupation and the Impact of Vaccinations
- PMID: 40573953
- PMCID: PMC12197333
- DOI: 10.3390/vaccines13060622
A Multi-Center Study on Sensitization to Thimerosal in North-Eastern Italy, 1997-2023: Prevalence, Risk Factors, the Role of Occupation and the Impact of Vaccinations
Abstract
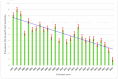
Background: Thimerosal has been widely used as a preservative to prevent microbial growth in medications and vaccines. However, in 1999 its removal from vaccine formulations was called for due to concerns about its potential side effects on humans, with subsequent reduced sensitizations at patch tests. The present multi-center study investigated the epidemiological, occupational and temporal pattern of sensitization to Thimerosal in North-Eastern Italy during 1997-2023 and associated factors. Methods: Due to variability in patch testing and positive reactions by the centers, this study was broken down by three periods: 1997-2004 (including all centers but Trieste); 1997-2015 (considering only Padua and Pordenone); and 2010-2023 (considering only Trieste and Pordenone). Multiple logistic regression was used to investigate prevalence of sensitization to Thimerosal and associated factors. Results were expressed as adjusted odds ratio (aOR) with 95% confidence intervals (95%CI). Results: Prevalence of positive patch test reactions to Thimerosal decreased from (8.13%) in 1997 to 0.95% in 2023 across all centers combined. Prevalence of positivity to Thimerosal was 9.49% during 1997-2004 (in all centers yet excluding Trieste), 8.41% during 1997-2015 (considering only Padua and Pordenone) and 4.01% during 2010-2023 (considering only Trieste and Pordenone). A significantly decreasing trend of Thimerosal sensitization was observed during 1997-2015 (aOR = 0.94; 95%CI: 0.92; 0.95). Regardless of the study period, sensitization to Thimerosal was consistently and significantly higher among health care workers (HCWs) and in patients born during 1981-1990. Conclusions: The significantly decreasing prevalence of sensitization to Thimerosal over time likely reflected removal policies from vaccines and medications after 1999. Likewise, the higher prevalence of patch test reactions in patients born during 1981-1990 may mirror the widespread presence of this hapten in vaccines and medications in the 1980ies. Moreover, the increased prevalence of patch test reactions positive to Thimerosal in HCWs probably reflected higher influenza vaccination uptake in this group compared to other occupational categories. Positive patch test reactions to Thimerosal after 2000 were likely clinically irrelevant though.
Keywords: Methiolate; Thimerosal; allergic contact dermatitis; allergology; patch test; prevalence; sensitization; standard series; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Center for Disease Control and Preventoin (CDC) Thimerosal in Flu Vaccines. [(accessed on 9 March 2025)]; Available online: https://www.cdc.gov/flu/vaccine-safety/thimerosal.html.
LinkOut - more resources
Full Text Sources