Development of an Influenza/COVID-19 Combination mRNA Vaccine Containing a Novel Multivalent Antigen Design That Enhances Immunogenicity of Influenza Virus B Hemagglutinins
- PMID: 40573959
- PMCID: PMC12197711
- DOI: 10.3390/vaccines13060628
Development of an Influenza/COVID-19 Combination mRNA Vaccine Containing a Novel Multivalent Antigen Design That Enhances Immunogenicity of Influenza Virus B Hemagglutinins
Abstract
Background/objectives: Developing next-generation mRNA-based seasonal influenza vaccines remains challenging, primarily because of the relatively low immunogenicity of influenza B hemagglutinin (HA) antigens. We describe a systematic vaccine development strategy that combined vector and antigen design optimization.
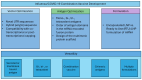
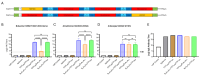
Methods: Novel untranslated region (UTR) sequences and a hybrid poly(A) tail were used to increase plasmid stability and mRNA expression. Fusion proteins containing HA antigens linked by T4 foldon domains were engineered to enhance the immune responses against influenza B HA antigens and to permit the expression of multiple HA ectodomains from a single mRNA species. The vaccine performance was verified in a traditional encapsulated lipid nanoparticle (LNP) formulation that requires long-term storage at temperatures below -15 °C as well as in a proprietary thermo-stable LNP formulation developed for the long-term storage of the mRNA vaccine at 2-8 °C.
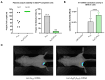
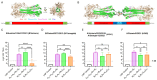
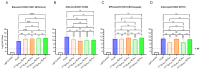
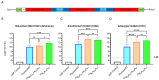
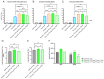
Results: In preclinical studies, our next-generation seasonal influenza vaccine tested alone or as a combination influenza/COVID-19 mRNA vaccine elicited hemagglutination inhibition (HAI) titers significantly higher than Fluzone HD, a commercial inactivated influenza vaccine, across all 2024/2025 seasonal influenza strains, including the B/Victoria lineage strain. At the same time, the combination mRNA vaccine demonstrated superior neutralizing antibody titers to 2023/2024 Spikevax, a commercial COVID-19 comparator mRNA vaccine.
Conclusions: Our data demonstrate that the multimerization of antigens expressed as complex fusion proteins is a powerful antigen design approach that may be broadly applied toward mRNA vaccine development.
Keywords: hemagglutinin; influenza/COVID-19 combination vaccine; multimeric antigens; seasonal influenza mRNA vaccine.
Conflict of interest statement
All authors are employees of Immorna Biotherapeutics, Inc. or Immorna Biotechnology, Co., Ltd. This affiliation had no influence on the design, execution, or interpretation of the study. This research is associated with a pending patent application filed by Immorna Biotherapeutics, Inc.
Figures
Similar articles
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Self-amplifying mRNA expressing COBRA hemagglutinin elicits long-lasting, broadly reactive antibodies against seasonal influenza A viruses.Vaccine. 2025 Jul 4;62:127449. doi: 10.1016/j.vaccine.2025.127449. Online ahead of print. Vaccine. 2025. PMID: 40617088
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
Vaccines for preventing influenza in healthy adults.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD001269. doi: 10.1002/14651858.CD001269.pub6. Cochrane Database Syst Rev. 2018. PMID: 29388196 Free PMC article.
References
-
- Centers for Disease Control and Prevention About Estimated Flu Burden. [(accessed on 21 February 2025)]; Available online: https://www.cdc.gov/flu-burden/php/about/index.html.
-
- Centers for Disease Control and Prevention Preliminary Estimated Flu Disease Burden 2024–2025 Flu Season. [(accessed on 21 February 2025)]; Available online: https://www.cdc.gov/flu-burden/php/data-vis/2024-2025.html.
-
- Centers for Disease Control and Prevention Weekly US Influenza Surveillance Report: Key Updates for Week 52, Ending 28 December 2024. [(accessed on 21 February 2025)]; Available online: https://www.cdc.gov/fluview/surveillance/2024-week-52.html.
-
- World Health Organization History of the Influenza Vaccine. [(accessed on 21 February 2025)]. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/history-o....
LinkOut - more resources
Full Text Sources

