Innovation in mRNA Vaccines and RNAi via Protein Nanocages
- PMID: 40573984
- PMCID: PMC12197727
- DOI: 10.3390/vaccines13060653
Innovation in mRNA Vaccines and RNAi via Protein Nanocages
Abstract
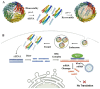
Self-assembling protein nanocages (SAPNs) are distinct natural structures formed by the self-assembly of identical subunits, providing a highly efficient platform and a novel strategy for vaccine development and RNAi therapy. Their internal cavity allows for precise cargo encapsulation, while the externally modifiable surface supports multivalent antigen presentation, thereby enhancing stability, targeted delivery, and immune activation. In addition to serving as stable subunit vaccines with multivalent antigen display, SAPNs can be incorporated into mRNA vaccines (SAPN-RNA vaccines) by pre-fusing with the antigen. This strategy stabilizes secreted antigenic proteins with prolonged presentation to the immune system, and improves vaccine efficacy while reducing off-target effects and minimizing required doses. Additionally, SAPNs can overcome cellular uptake barriers, enhance DNA vaccine efficacy, and enable the co-delivery of antigens and adjuvants. Functionalization with adjuvants or targeting ligands further improves their immunostimulatory properties and specificity. The SAPN-RNAi strategy optimizes siRNA delivery by promoting lysosomal escape, enhancing targeted uptake, and protecting siRNA from degradation through SAPN encapsulation. This review examines the structural and functional properties of protein nanocages and their applications in vaccine design and RNAi delivery, emphasizing their synergistic effects, and exploring current progress, challenges, and future directions. In conclusion, SAPNs represent a versatile multifunctional platform with broad applicability across subunit, mRNA and DNA vaccines, adjuvant co-delivery, and RNAi therapeutics, with significant potential against viral infections.
Keywords: ferritin; mRNA vaccines; protein nanocages; siRNA delivery; vaccine platform; virus.
Conflict of interest statement
The author declares no conflict of interest.
Figures


Similar articles
-
Accreditation through the eyes of nurse managers: an infinite staircase or a phenomenon that evaporates like water.J Health Organ Manag. 2025 Jun 30. doi: 10.1108/JHOM-01-2025-0029. Online ahead of print. J Health Organ Manag. 2025. PMID: 40574247
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
The use of Open Dialogue in Trauma Informed Care services for mental health consumers and their family networks: A scoping review.J Psychiatr Ment Health Nurs. 2024 Aug;31(4):681-698. doi: 10.1111/jpm.13023. Epub 2024 Jan 17. J Psychiatr Ment Health Nurs. 2024. PMID: 38230967
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
Harnessing flagellin of Ligilactobacillus agilis as a surface display scaffold for an HIV-1 epitope.Appl Environ Microbiol. 2025 Jun 18;91(6):e0067425. doi: 10.1128/aem.00674-25. Epub 2025 May 29. Appl Environ Microbiol. 2025. PMID: 40439423 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

