Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies
- PMID: 40573996
- PMCID: PMC12196053
- DOI: 10.3390/pharmaceutics17060682
Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies
Abstract
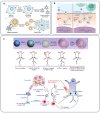
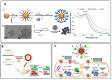
Cancer remains a formidable global health challenge due to its complex pathophysiology and resistance to conventional treatments. In recent years, the convergence of nanotechnology and oncology has paved the way for innovative therapeutic platforms that address the limitations of traditional modalities. This review examines how nanoparticle (NP)-based strategies enhance the efficacy of chemotherapy, radiotherapy, phototherapy, immunotherapy, and gene therapy by enabling targeted delivery, controlled drug release, and tumor-specific accumulation via the enhanced permeability and retention (EPR) effect. We discuss the design and functionalization of various organic, inorganic, and hybrid NPs, highlighting their roles in improving pharmacokinetics, overcoming multidrug resistance, and modulating the tumor microenvironment. Particular emphasis is placed on dual and multimodal therapies, such as chemo-phototherapy, chemo-immunotherapy, and gene-radiotherapy, that leverage nanoparticle carriers to amplify synergistic effects, minimize systemic toxicity, and improve clinical outcomes. We also explore cutting-edge advances in gene editing and personalized nanomedicine, as well as emerging strategies to address biological barriers and immunosuppressive mechanisms in the tumor niche. Despite the undeniable promise of nanoparticle-based cancer therapies, challenges related to toxicity, scalable manufacturing, regulatory oversight, and long-term biocompatibility must be overcome before they can fully enter clinical practice. By synthesizing recent findings and identifying key opportunities for innovation, this review provides insight into how nanoscale platforms are propelling the next generation of precision oncology.
Keywords: multimodal treatment; nanoparticles; personalized oncology; synergistic cancer therapy; targeted drug delivery; translational nanomedicine; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
How to Implement Digital Clinical Consultations in UK Maternity Care: the ARM@DA Realist Review.Health Soc Care Deliv Res. 2025 May;13(22):1-77. doi: 10.3310/WQFV7425. Health Soc Care Deliv Res. 2025. PMID: 40417997 Review.
-
[Narrow-band UVB therapy in psoriasis vulgaris: good practice guideline and recommendations of the French Society of Photodermatology].Ann Dermatol Venereol. 2010 Jan;137(1):21-31. doi: 10.1016/j.annder.2009.12.004. Epub 2009 Dec 29. Ann Dermatol Venereol. 2010. PMID: 20110064 French.
-
Nanoparticle technologies in precision oncology and personalized vaccine development: Challenges and advances.Int J Pharm X. 2025 Jul 5;10:100353. doi: 10.1016/j.ijpx.2025.100353. eCollection 2025 Dec. Int J Pharm X. 2025. PMID: 40688030 Free PMC article. Review.
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Mechanistic insight into nanomedicine for polycystic ovary syndrome.Mol Biol Rep. 2025 Jun 21;52(1):618. doi: 10.1007/s11033-025-10709-7. Mol Biol Rep. 2025. PMID: 40544202 Review.
References
-
- Cooper G., Hausman R. The development and causes of cancer. Cell Mol. Approach. 2000;2:725–766.
-
- Torok J.A., Wu Y., Chino J., Prosnitz L.R., Beaven A.W., Kim G.J., Kelsey C.R. Chemotherapy or combined modality therapy for early-stage Hodgkin lymphoma. Anticancer Res. 2018;38:2875–2881. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

