pH-Responsive Liposome-Hydrogel Composite Accelerates Nasal Mucosa Wound Healing
- PMID: 40574003
- PMCID: PMC12195687
- DOI: 10.3390/pharmaceutics17060690
pH-Responsive Liposome-Hydrogel Composite Accelerates Nasal Mucosa Wound Healing
Abstract
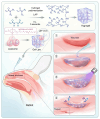
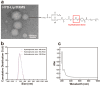
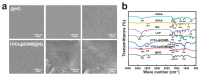
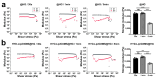
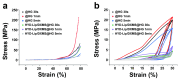
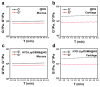
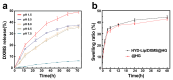
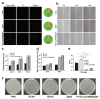
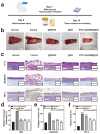
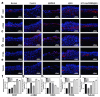
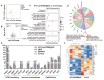
Objectives: Nasal mucosa wound healing faces challenges such as acidic microenvironments and bacterial proliferation. Persistent mucosal defects predispose to complications such as nasal septal perforation. Conventional drug delivery systems suffer from nonspecific release and short-term efficacy. This study aimed to develop a pH-responsive liposome-hydrogel composite (HYD-Lip/DXMS@HG) to integrate pH-triggered dexamethasone (DXMS) delivery, antifouling properties, and mechanical support for refractory injuries. Methods: The composite combined acylhydrazone-modified liposomes with a hydrogel synthesized from hydroxyethylacrylamide (HEAA) and diethylacrylamide (DEAA). In vitro assays evaluated DXMS release kinetics, RPMI 2650 cell migration/proliferation, and antibacterial properties. In vivo rabbit nasal mucosal injury models assessed healing efficacy via histology analyses. RNA sequencing was performed to identify key signaling pathways. Results: HYD-Lip/DXMS@HG exhibited sustained DXMS release in acidic conditions, accelerating cell migration/proliferation in vitro. In rabbits, the composite reduced TNF-α expression and CD45+ leukocyte infiltration, while enhancing collagen alignment and epithelial thickness. RNA sequencing identified upregulated ECM receptor interaction, Hippo, TGF-β, and PI3K-Akt pathways, linked to collagen remodeling, anti-apoptosis, and angiogenesis. Conclusions: This multifunctional platform synergizes pH-triggered drug delivery, mechanical support, and antibacterial activity, offering a promising therapeutic strategy for refractory nasal mucosal injuries and postoperative recovery.
Keywords: acylhydrazone bond; antibacterial; dexamethasone; hydrogel; liposome; nasal mucosa; pH-responsive.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats.J Orthop Translat. 2025 Jun 5;53:63-81. doi: 10.1016/j.jot.2025.04.015. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40529900 Free PMC article.
-
Poly(ethylene glycol)-norbornene-heparin-piposome composite hydrogels for in situ spraying and ultra-fast adhesion: meeting the challenges of endothelial repair of vascular injury.Acta Biomater. 2025 Jun 15;200:489-507. doi: 10.1016/j.actbio.2025.04.060. Epub 2025 May 15. Acta Biomater. 2025. PMID: 40381928
-
A biomimetic nanofiber composite hydrogel with tissue adhesion, self-healing and antibacterial ability for infected wound healing.Acta Biomater. 2025 Jun 15;200:326-339. doi: 10.1016/j.actbio.2025.04.002. Epub 2025 Apr 2. Acta Biomater. 2025. PMID: 40185462
-
Hydrogel dressings for venous leg ulcers.Cochrane Database Syst Rev. 2022 Aug 5;8(8):CD010738. doi: 10.1002/14651858.CD010738.pub2. Cochrane Database Syst Rev. 2022. PMID: 35930364 Free PMC article.
-
Measurement of pH, exudate composition and temperature in wound healing: a systematic review.J Wound Care. 2017 Jul 2;26(7):381-397. doi: 10.12968/jowc.2017.26.7.381. J Wound Care. 2017. PMID: 28704150
References
-
- Seefeld M.L., Templeton E.L., Lehtinen J.M., Sinclair N., Yadav D., Hartwell B.L. Harnessing the potential of the NALT and BALT as targets for immunomodulation using engineering strategies to enhance mucosal uptake. Front. Immunol. 2024;15:1419527. doi: 10.3389/fimmu.2024.1419527. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

