Improving Pulmonary Delivery of Budesonide Suspensions Nebulized with Constant-Output Vibrating Mesh Nebulizers by Using Valved Holding Chamber
- PMID: 40574009
- PMCID: PMC12196008
- DOI: 10.3390/pharmaceutics17060696
Improving Pulmonary Delivery of Budesonide Suspensions Nebulized with Constant-Output Vibrating Mesh Nebulizers by Using Valved Holding Chamber
Abstract
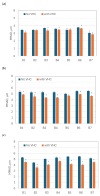
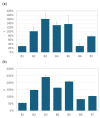
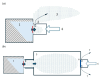
Background: Vibrating mesh nebulizers (VMNs) are not only used to deliver typical pulmonary drugs but are also a promising platform for novel formulations and therapeutic applications. Typically, these devices operate continuously or on demand and are directly connected to the outflow interface (mouthpiece or mask) without valving systems that could spare the drug during exhalation. This paper examines the possibility of increasing the delivery of inhaled budesonide aerosol by attaching a valved holding chamber (VHC) to selected VMNs. Methods: A laboratory in vitro study was conducted for seven budesonide (BUD) nebulization products (0.25 mg/mL). The rates of aerosol delivery from VMNs alone or VMN + VHC systems were determined gravimetrically for a simulated breathing cycle, while droplet size distributions in mists were measured by laser diffraction. Results: The VMN + VHC systems increased the amount of aerosol available for inhalation and the fraction of fine particles that could penetrate the pulmonary region. Depending on the VMN and BUD product, a relative increase of 30-300% in the total drug delivery (T) and 50-350% in the pulmonary drug availability (P) was obtained. The results are explained by the reduction in aerosol losses during exhalation (the fugitive emission) by the VHC and the simultaneous elimination of the largest droplets due to coalescence and deposition in the chamber. Both VMN and BUD affected the aerosol's properties and discharge mass and thus the actual benefits of the VHC. Conclusions: While the results confirm the superiority of VMN + VHC over VMNs alone in nebulizing BUD suspensions, they also show that it is difficult to predict the effects quantitatively without testing the individual nebulizer-chamber-drug combination.
Keywords: aerosol; budesonide; drug availability; inhalation; nebulization.
Conflict of interest statement
The authors declare no conflicts of interest regarding this study.
Figures






Similar articles
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
In Vitro Comparison of Inspiration-Synchronized and Continuous Vibrating Mesh Nebulizer During Adult Invasive Mechanical Ventilation.J Aerosol Med Pulm Drug Deliv. 2025 Apr;38(2):64-70. doi: 10.1089/jamp.2024.0047. Epub 2024 Dec 9. J Aerosol Med Pulm Drug Deliv. 2025. PMID: 39648821
-
Aerosol Delivery to Simulated Spontaneously Breathing Tracheostomized Adult Model With and Without Humidification.Respir Care. 2024 Jun 28;69(7):847-853. doi: 10.4187/respcare.11495. Respir Care. 2024. PMID: 38485144 Free PMC article.
-
A Pediatric Bench Model of Continuous Albuterol Delivery Using Heliox.Respir Care. 2024 Nov 18;69(12):1517-1522. doi: 10.4187/respcare.11713. Respir Care. 2024. PMID: 39137952
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.Health Technol Assess. 2008 May;12(19):iii-iv, 1-360. doi: 10.3310/hta12190. Health Technol Assess. 2008. PMID: 18485271
References
-
- Martin A.R. Regional deposition: Targeting. J. Aerosol Med. Pulm. Drug Deliv. 2021;34:1–10. doi: 10.1089/jamp.2021.29033.am. - DOI - PubMed
LinkOut - more resources
Full Text Sources

