Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats
- PMID: 40574016
- PMCID: PMC12196312
- DOI: 10.3390/pharmaceutics17060703
Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats
Abstract
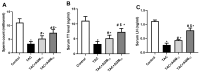
Background/Objectives: Tacrolimus is an immunosuppressant drug widely used to prevent organ transplant rejection. Preclinical and clinical studies report that tacrolimus has destructive impacts on the male reproductive system owing to the induction of oxidative stress and inflammation. This study aimed at examining defensive impacts of agmatine against tacrolimus-induced testicular toxicity in rats. Methods: Male Wistar rats were randomly divided into six groups and treated based on the experimental design for 14 days. By the end of this study, blood samples were obtained to measure testosterone and luteinizing hormone. Also, both testes were removed for molecular analysis and histopathological examinations. Results: Agmatine administration increased serum levels of testosterone and luteinizing hormone and ameliorated all histopathological and toxicological changes induced by tacrolimus. Agmatine administration attenuated tacrolimus-induced oxidative stress as evidenced by the reduction of malondialdehyde content and inducible nitric oxide synthase expression and the elevation of reduced glutathione. This was parallel to the restoration of nuclear factor erythroid 2-related factor2 and hemeoxygenase-1 expression. Moreover, agmatine decreased the expressions of nuclear factor kappa B and interleukin-17. Agmatine also decreased the cell death revealed by decreased caspase-3 expression and increased expression of the antiapoptotic marker Bcl-2 in a dose-dependent manner. The antioxidant, anti-inflammatory, and antiapoptotic effects of agmatine were explained by increased expression of sirtuin-1. Conclusions: agmatine effectively attenuated testicular injuries induced by tacrolimus and enhanced spermatogenesis. This protective effect of agmatine might be mediated via the upregulation of sirtuin-1 expression that in turn restores oxidative status and regulates nuclear factor erythroid 2-related factor2/nuclear factor kappa B/Bcl-2 signaling.
Keywords: agmatine; oxidative stress; tacrolimus; testicular toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Morin hydrate protects against cisplatin-induced testicular toxicity by modulating ferroptosis and steroidogenesis genes' expression and upregulating Nrf2/Heme oxygenase-1.Sci Rep. 2025 Jul 2;15(1):22720. doi: 10.1038/s41598-025-08235-4. Sci Rep. 2025. PMID: 40595313 Free PMC article.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.Health Technol Assess. 2005 May;9(21):1-179, iii-iv. doi: 10.3310/hta9210. Health Technol Assess. 2005. PMID: 15899149
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.Health Technol Assess. 2006 Dec;10(49):iii-iv, ix-xi, 1-157. doi: 10.3310/hta10490. Health Technol Assess. 2006. PMID: 17134597
-
Melatonin as a potential adjuvant to mitigate depakine‑induced testicular damage in rats through its biological features.BMC Complement Med Ther. 2025 Jun 25;25(1):213. doi: 10.1186/s12906-025-04969-w. BMC Complement Med Ther. 2025. PMID: 40563113 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
LinkOut - more resources
Full Text Sources
Research Materials

