Assessment of Innovative Dry Powders for Inhalation of a Synergistic Combination Against Mycobacterium tuberculosis in Infected Macrophages and Mice
- PMID: 40574018
- PMCID: PMC12195745
- DOI: 10.3390/pharmaceutics17060705
Assessment of Innovative Dry Powders for Inhalation of a Synergistic Combination Against Mycobacterium tuberculosis in Infected Macrophages and Mice
Abstract
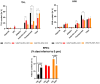
Background/Objectives: In vitro, vancomycin (VAN) and tetrahydrolipstatin (THL) together have been shown to synergistically inhibit Mycobacterium tuberculosis (Mtb), the world's most infectious killer. The poor oral bioavailability of VAN and THL and predominant tropism of Mtb infection to the lungs and alveolar macrophages make pulmonary administration highly attractive. This study aimed to develop and assess the efficacy of dry powders for inhalation of VAN microparticles embedded with THL. Methods: The dry powders produced by spray-drying, with or without hydrogenated castor oil (HCO), were characterized for their physicochemical properties among others by HPLC-DAD. The fast-screening impactor was used to determine powder aerodynamic properties, and VAN and THL releases were established from the paddle over disk method. Biological activities were assessed in a new M. bovis-infected macrophage model and in Mtb-infected mice. Results and Discussion: The addition of 25% HCO enables co-deposition (fine particle dose) at the desired weight ratio and co-releasing of VAN and THL in aqueous media. Microparticles with 0% to 50% HCO drastically reduced cytoplasmic Mycobacterium bovis survival (99.9% to 62.5%, respectively), with higher efficacy at low HCO concentration. Consequently, VAN/THL with or without 25% HCO was evaluated in Mtb-infected mice. Although no decrease in Mtb lung burden was observed after two weeks of administration, the endotracheal administration of VAN 500 mg/kg and THL 50 mg/kg with 25% HCO administrated three times during five days concomitantly with daily oral rifampicin (10 mg/kg) demonstrated 2-fold bacterial burden reduction compared to the group treated with RIF alone. Conclusions: HCO was crucial for obtaining a fine particle dose at the synergistic weight ratio (VAN/THL 10:1) and for releasing both drugs in aqueous media. With oral administration of the first-line rifampicin, the dry powder VAN/THL/25% HCO was able to exert a potential anti-tubercular effect in vivo in Mtb-infected mice after five days.
Keywords: macrophage infection model; orlistat; pulmonary delivery; tuberculosis; vancomycin.
Conflict of interest statement
Faustine Ravon, Emilie Berns, Isaline Lambert, Céline Rens, Pierre-Yves Adnet, Mehdi Kiass, Véronique Megalizzi, Cedric Delporte, Alain R. Baulard, Vanessa Mathys and Samira Boarbi declare no conflicts of interest. Véronique Fontaine is involved at 50% as inventor in a European patent, N° EP 3 237 011 B1, covering the discovery of a synergy between tetrahydrolipstatin (Orlistat) and vancomycin (reference: WO 2016/102541, 30.06.2016 Gazette 2016/26). Nathalie Wauthoz is involved at 33% as inventor in a patent covering a formulation based on HCO: “K. Amighi, N. Wauthoz, R. Rosière. Dry powder inhalation formulation and its use for the therapeutic treatment of lungs”. Date of publication: 28th December 2019, International number: PCT/EP2019/087122, Publication number: WO 2020/136276.
Figures
Similar articles
-
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children.Cochrane Database Syst Rev. 2022 Sep 6;9(9):CD013359. doi: 10.1002/14651858.CD013359.pub3. Cochrane Database Syst Rev. 2022. PMID: 36065889 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
References
-
- World Health Organization Global Tuberculosis Report 2024. [(accessed on 7 March 2025)]. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa....
-
- Raman S.K., Roy T., Verma K., Yadav C., Verma S., Deivreddy V.S.R., Sofi H.S., Bharti R., Sharma R., Bansode H., et al. Dry powder Inhalation of lytic mycobacteriophages for adjunct therapy in a mouse model of infection with Mycobacterium tuberculosis. Tuberculosis. 2025;152:102631. doi: 10.1016/j.tube.2025.102631. - DOI - PubMed
-
- Laohapojanart N., Ratanajamit C., Kawkitinarong K., Srichana T. Efficacy and safety of combined isoniazid-rifampicin-pyrazinamide-levofloxacin dry powder inhaler in treatment of pulmonary tuberculosis: A randomized controlled trial. Pulm. Pharmacol. Ther. 2021;70:102056. doi: 10.1016/j.pupt.2021.102056. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

