Design of Tranilast-Loaded Cation-Type Contact Lens for Sustainable Ocular Drug Delivery
- PMID: 40574025
- PMCID: PMC12196024
- DOI: 10.3390/pharmaceutics17060712
Design of Tranilast-Loaded Cation-Type Contact Lens for Sustainable Ocular Drug Delivery
Abstract
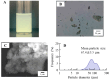
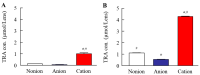
Objectives: This study evaluated the design of a sustained-release contact lens (CL) device loading tranilast (TRA) and determined the usefulness of these CLs in Japanese albino rabbits. Methods: The sustainable CLs in this study were prepared by combining three CLs with different water contents and soaking methods under high-pressure and high-temperature using an autoclave method (AC-method). Results: Both the CLs prepared with the conventional soaking method (stir-method) and AC-methods were transparent in all three types of CLs. The loaded TRA contents in the CLs when using the AC-method were higher than those prepared using the stir-method for all three types of CLs. TRA contents were also higher when loaded into the cation-type lenses as compared to the other lenses. Moreover, the sustainable release of TRA from the TRA-loaded cation-type CL using the AC-method was significantly higher than those found for the other CLs. No corneal wounds were observed in any of the rabbits given the three types of TRA-loaded CLs for 7 days. Furthermore, the TRA-loaded CL sustainably released TRA into the lacrimal fluid in the rabbit. Conclusions: The TRA-loaded CL prepared using the AC-method overcame the limitations normally associated with the stir-method, such as the high burst release and low drug uptake.
Keywords: autoclave; cation; contact lenses; lacrimal fluid; tranilast.
Conflict of interest statement
Authors Toru Matsunaga and Shiori Hino are affiliated with a company SEED Co., Ltd. but have no potential conflicts of interest relating to this relationship. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures




Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Yoga for epilepsy.Cochrane Database Syst Rev. 2017 Oct 5;10(10):CD001524. doi: 10.1002/14651858.CD001524.pub3. Cochrane Database Syst Rev. 2017. PMID: 28982217 Free PMC article.
-
Interventions for recurrent corneal erosions.Cochrane Database Syst Rev. 2018 Jul 9;7(7):CD001861. doi: 10.1002/14651858.CD001861.pub4. Cochrane Database Syst Rev. 2018. PMID: 29985545 Free PMC article.
-
Dietary interventions for recurrent abdominal pain in childhood.Cochrane Database Syst Rev. 2017 Mar 23;3(3):CD010972. doi: 10.1002/14651858.CD010972.pub2. Cochrane Database Syst Rev. 2017. PMID: 28334433 Free PMC article.
References
-
- Kumar A., Malviya R., Sharma P. Recent trends in ocular drug delivery: A short review. Am. J. Appl. Sci. 2011;3:86–92.
Grants and funding
LinkOut - more resources
Full Text Sources

