Impact of Different Hydrate Forms of Magnesium Stearate as a Flow Control Agent on the Physical Stability and Inhalation Efficiency of Carrier-Based Formulations
- PMID: 40574026
- PMCID: PMC12195998
- DOI: 10.3390/pharmaceutics17060711
Impact of Different Hydrate Forms of Magnesium Stearate as a Flow Control Agent on the Physical Stability and Inhalation Efficiency of Carrier-Based Formulations
Abstract
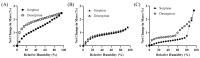
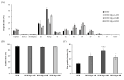
Objectives: This study aimed to evaluate the impact of the different hydration states of magnesium stearate (Mg.st) anhydrate (AH), monohydrate (MH), and dihydrate (DH) on the aerodynamic performance and stability of carrier-based dry powder inhalation (DPI) formulations using arformoterol and budesonide as model drugs. Methods: DPI formulations were prepared using Inhalac 251 lactose and Mg.st in various hydrated forms. The physicochemical properties of Mg.st were characterized using powder X-ray diffraction, differential scanning calorimetry, Fourier-transform infrared spectroscopy, Karl Fischer titration, dynamic vapor absorption, and Raman imaging. The aerodynamic performance was assessed employing a next-generation impactor under initial and accelerated conditions (40 °C, 75% relative humidity). Results: Mg.st-MH exhibited the highest crystallinity and the most stable moisture sorption profile, and showed the smallest particle size within the formulation as observed in the Raman images. Formulations containing Mg.st-MH demonstrated significantly higher fine particle fractions for both arformoterol (51.02 ± 5.16%) and budesonide (61.98 ± 4.09%) compared to formulations with Mg.st-AH or -DH forms. Mg.st-MH also exhibited improved performance retention under accelerated conditions, correlating with its physicochemical stability. Conclusions: The monohydrate form of magnesium stearate was the most effective force control agent, which reduced interparticulate interactions, thereby enhancing the inhalation efficiency and formulation stability. Thus, selecting an appropriate hydration form of Mg.st can improve DPI performance.
Keywords: aerodynamic performance; carrier based dry powder inhalation; force control agent; hydrate form; magnesium monohydrate.
Conflict of interest statement
Jaewoon Son was employed by GC Biopharma, and Chang-Soo Han was employed by P2KBio. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The companies had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Begat P., Price R., Harris H., Morton D.A., Staniforth J.N. The Influence of force control agents on the cohesive-adhesive balance in dry powder inhaler formulations. KONA. 2005;23:109–121. doi: 10.14356/kona.2005014. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

