Mechanistic Insight into the Enhanced Anti-Pulmonary Hypertension Efficacy of Wogonin Co-Amorphous
- PMID: 40574037
- PMCID: PMC12196166
- DOI: 10.3390/pharmaceutics17060724
Mechanistic Insight into the Enhanced Anti-Pulmonary Hypertension Efficacy of Wogonin Co-Amorphous
Abstract
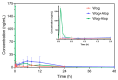
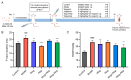
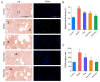
Background: Pulmonary hypertension (PH) remains a life-threatening rare disease characterized by inflammation and oxidative stress in pulmonary artery smooth muscle cells (PASMCs). Wogonin (Wog), a plant-derived polyphenolic compound extracted from Scutellaria baicalensis Georgi, exhibits notable antioxidant activity and anti-PH efficacy, whereas its clinical applications are greatly limited by poor aqueous solubility. Methods: Herein, an innovative wogonin-aloperine co-amorphous (Wog-Alop) was developed to improve the aqueous solubility and, thus, anti-PH efficacy of Wog. Results: As expected, the aqueous solubility of Wog-Alop is about 40-fold that of Wog; meanwhile, the Wog-Alop demonstrates better oral bioavailability and anti-PH efficacy than Wog; moreover, the Wog-Alop exhibits significantly enhanced capacity to attenuate oxidative stress in human PASMCs compared to Wog. Conclusions: The results suggested that Wog-Alop could not only improve the solubility of Wog, thereby enhancing its oral bioavailability but also alleviate Wog's oxidative stress effects. These synergistic effects ultimately culminate in the enhanced anti-PH efficacy of Wog. In summary, the present study developed an innovative co-amorphous strategy for the delivery of Wog and improved its anti-PH efficacy.
Keywords: co-amorphous; oxidative stress; pulmonary hypertension; solubility; wogonin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Cueto-Robledo G., Tovar-Benitez D., Alfaro-Cruz A., Gonzalez-Hermosillo L.-M. Systemic Scleroderma: Review and Updated Approach and Case Description to Addressing Pulmonary Arterial Hypertension and Idiopathic Pulmonary Fibrosis: A Dual Challenge in Treatment. Curr. Probl. Cardiol. 2024;49:102404. doi: 10.1016/j.cpcardiol.2024.102404. - DOI - PubMed
-
- Pugliese S.C., Poth J.M., Fini M.A., Olschewski A., El Kasmi K.C., Stenmark K.R. The Role of Inflammation in Hypoxic Pulmonary Hypertension: From Cellular Mechanisms to Clinical Phenotypes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L229–L252. doi: 10.1152/ajplung.00238.2014. - DOI - PMC - PubMed
-
- Chen J.N., Zhou R.D.P. On the Sign-Imbalance of Permutation Tableaux. Adv. Appl. Math. 2017;86:1–18. doi: 10.1016/j.aam.2016.11.012. - DOI
-
- Zimmer A., Teixeira R.B., Constantin R.L., Campos-Carraro C., Aparicio Cordero E.A., Ortiz V.D., Donatti L., Gonzalez E., Bahr A.C., Visioli F., et al. The Progression of Pulmonary Arterial Hypertension Induced by Monocrotaline Is Characterized by Lung Nitrosative and Oxidative Stress, and Impaired Pulmonary Artery Reactivity. Eur. J. Pharmacol. 2021;891:173699. doi: 10.1016/j.ejphar.2020.173699. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

