Novel Polymorphic Patterns for Elacestrant Dihydrochloride
- PMID: 40574057
- PMCID: PMC12197126
- DOI: 10.3390/pharmaceutics17060745
Novel Polymorphic Patterns for Elacestrant Dihydrochloride
Abstract
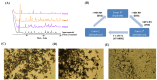
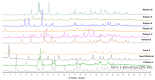
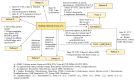
Objective: This study expands on the polymorphic characterization of elacestrant dihydrochloride, developed by Stemline Therapeutics and approved by the FDA in 2023. The article focuses on more extensive polymorphism screening using various methods and solvents to discover the new polymorphism forms of this molecule, besides identifying three polymorphic forms in the previously published studies. Methods: The crystalline and amorphous elacestrant hydrochloride solubility was assessed, and crystals were formed, followed by polymorph screening using 40 non-conventional solvents via different techniques to obtain the new polymorphic forms. XRPD, NMR, DSC, TGA, IC, and HPLC were used for solid-state characterization. Results: Patterns A, B, C, D, E, F, and G, and previously published forms 1,3, were identified in multiple studies during the extensive polymorphism screening using various methods and numerous solvent systems. Solid state characterization and purity analysis were completed using different relevant instruments. After the characterization, it was found that Pattern A was the most stable, like the desired/most stable Form 1, but it had fewer crystals; Pattern B is like Form 3 but a unique XRPD pattern; Pattern D is degradant; Pattern C, E, F, and G are considered as the new pattern of elacestrant along with patterns A and B. Conclusions: With XRPD, six new patterns (A, B, C, E, F, G) were identified. Patterns A, C, and E are promising crystalline candidates for further analysis and scale-up.
Keywords: amorphous; anti-solvent addition; characterization; crystalline; elacestrant; polymer templating; polymorphism; solvent-drop grinding.
Conflict of interest statement
P.G.S. and M.D.B.were employed by Stemline Therapeutics, Inc. V.G. was supported by a research contract grant by Stemline Therapeutics. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



References
-
- Bizuayehu H.M., Ahmed K.Y., Kibret G.D., Dadi A.F., Belachew S.A., Bagade T., Tegegne T.K., Venchiarutti R.L., Kibret K.T., Hailegebireal A.H., et al. Global Disparities of Cancer and Its Projected Burden in 2050. JAMA Netw. Open. 2024;7:e2443198. doi: 10.1001/jamanetworkopen.2024.43198. - DOI - PMC - PubMed
-
- Cancer Statistics—NCI. [(accessed on 7 January 2025)]; Available online: https://www.cancer.gov/about-cancer/understanding/statistics.
-
- Yara D., Oroszi T. Understanding Breast Cancer: A Comprehensive Review of Epidemiology, Risk Factors, and Treatment Strategies. Adv. Breast Cancer Res. 2025;14:1–15. doi: 10.4236/abcr.2025.141001. - DOI
LinkOut - more resources
Full Text Sources

